|
Tolrestat
Tolrestat (INN) (AY-27773) is an aldose reductase inhibitor which was approved for the control of certain diabetic complications. While it was approved for marketed in several countries, it failed a Phase III trial in the U.S. due to toxicity and never received FDA approval. It was discontinued by Wyeth Wyeth, LLC was an American pharmaceutical company. The company was founded in Philadelphia, Pennsylvania, in 1860 as ''John Wyeth and Brother''. It was later known, in the early 1930s, as American Home Products, before being renamed to Wyeth in ... in 1997 because of the risk of severe liver toxicity and death. It was sold under the tradename Alredase. References Aldose reductase inhibitors Acetic acids Hepatotoxins Naphthol ethers Trifluoromethyl compounds Thioamides Withdrawn drugs {{gastrointestinal-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldose Reductase Inhibitor
Aldose reductase inhibitors are a class of drugs being studied as a way to prevent eye and nerve damage in people with diabetes. Mechanism Their target, aldose reductase, is an enzyme that is normally present in many other parts of the body, and catalyzes one of the steps in the polyol pathway, sorbitol(polyol) pathway that is responsible for fructose formation from glucose. Aldose reductase activity increases as the glucose concentration rises in diabetes Diabetes, also known as diabetes mellitus, is a group of metabolic disorders characterized by a high blood sugar level ( hyperglycemia) over a prolonged period of time. Symptoms often include frequent urination, increased thirst and increased ... in those tissues that are not insulin sensitive, which include the lens (anatomy), lenses, peripheral nerves and glomerulus. Sorbitol does not diffuse through cell membranes easily and therefore accumulates, causing osmotic damage which leads to retinopathy and neuropathy. Examp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldose Reductase Inhibitors
Aldose reductase inhibitors are a class of drugs being studied as a way to prevent eye and nerve damage in people with diabetes. Mechanism Their target, aldose reductase, is an enzyme that is normally present in many other parts of the body, and catalyzes one of the steps in the sorbitol(polyol) pathway that is responsible for fructose formation from glucose. Aldose reductase activity increases as the glucose concentration rises in diabetes in those tissues that are not insulin sensitive, which include the lenses, peripheral nerves and glomerulus. Sorbitol does not diffuse through cell membranes easily and therefore accumulates, causing osmotic damage which leads to retinopathy and neuropathy. Examples File:Alrestatin.svg, Alrestatin File:Epalrestat.svg, Epalrestat File:Fidarestat structure.svg, Fidarestat File:Imirestat.svg, Imirestat File:Lidorestat.svg, Lidorestat File:Minalrestat.svg, Minalrestat File:Ponalrestat.svg, Ponalrestat File:Ranirestat.svg, Ranirestat File:Salfr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wyeth
Wyeth, LLC was an American pharmaceutical company. The company was founded in Philadelphia, Pennsylvania, in 1860 as ''John Wyeth and Brother''. It was later known, in the early 1930s, as American Home Products, before being renamed to Wyeth in 2002. Its headquarters moved to Collegeville, Pennsylvania and Madison, New Jersey, before they were consolidated with Pfizer's in New York City after the 2009 merger. Most of Wyeth's pharmaceutical assets were acquired by Pfizer in 2009, while its infant and maternal nutrition business was acquired by Nestlé in 2012. Wyeth manufactured over-the-counter drugs (OTCs) Robitussin and the analgesic Advil (ibuprofen) as well as prescription drugs Premarin and Effexor. History 1860–1899 In 1860, pharmacists John (1834–1907) and Frank Wyeth opened a drugstore with a small research lab on Walnut Street in Philadelphia. In 1862, on the suggestion of doctors, they began to manufacture large quantities of commonly ordered medicines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acids
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats. The global demand for acetic acid is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hepatotoxins
Hepatotoxicity (from ''hepatic toxicity'') implies chemical-driven liver damage. Drug-induced liver injury is a cause of acute and chronic liver disease caused specifically by medications and the most common reason for a drug to be withdrawn from the market after approval. The liver plays a central role in transforming and clearing chemicals and is susceptible to the toxicity from these agents. Certain medicinal agents, when taken in overdoses (e.g. paracetamol) and sometimes even when introduced within therapeutic ranges (e.g. halothane), may injure the organ. Other chemical agents, such as those used in laboratories and industries, natural chemicals (e.g., microcystins), and herbal remedies (two prominent examples being kava, mechanism unknown, and comfrey, through its pyrrolizidine alkaloid content) can also induce hepatotoxicity. Chemicals that cause liver injury are called hepatotoxins. More than 900 drugs have been implicated in causing liver injury (see LiverTox, externa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthol Ethers
Naphthol may refer to: * 1-Naphthol 1-Naphthol, or α-naphthol, is a fluorescent organic compound with the formula . It is a white solid. It is an isomer of 2-naphthol differing by the location of the hydroxyl group on the naphthalene ring. The naphthols are naphthalene homolog ... * 2-Naphthol {{Short pages monitor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
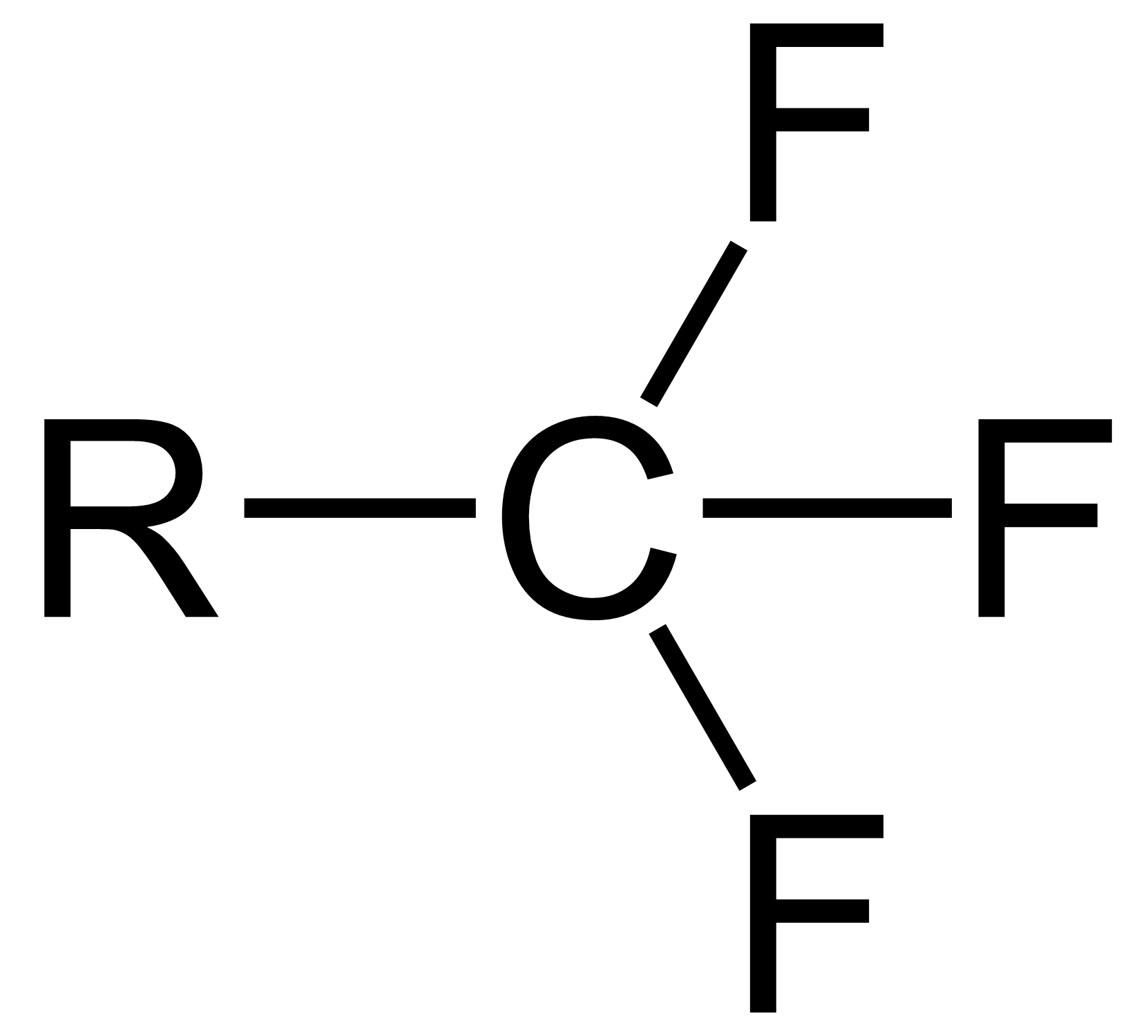
Trifluoromethyl Compounds
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone –CO–. Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioamides
A thioamide (rarely, thionamide, but also known as thiourylenes) is a functional group with the general structure R–CS–NR′R″, where R, R′, and R″ are organic groups. They are analogous to amides but they exhibit greater multiple bond character along the C-N bond, resulting in a larger rotational barrier. One of the best-known thioamides is thioacetamide, which is used as a source of the sulfide ion and is a building block in heterocyclic chemistry. Thioamides or anti-thyroid drugs are also a class of drugs that are used to control thyrotoxicosis. Preparation and structure Thioamides are typically prepared by treating amides with phosphorus sulfides such as phosphorus pentasulfide, a reaction first described in the 1870s. Alternative routes include the use of Lawesson's reagent or the reaction of nitriles with hydrogen sulfide: The Willgerodt-Kindler reaction also affords benzylthioamides. The C2NH2S core of thioamides is planar. Using thioacetamide as represent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |