|
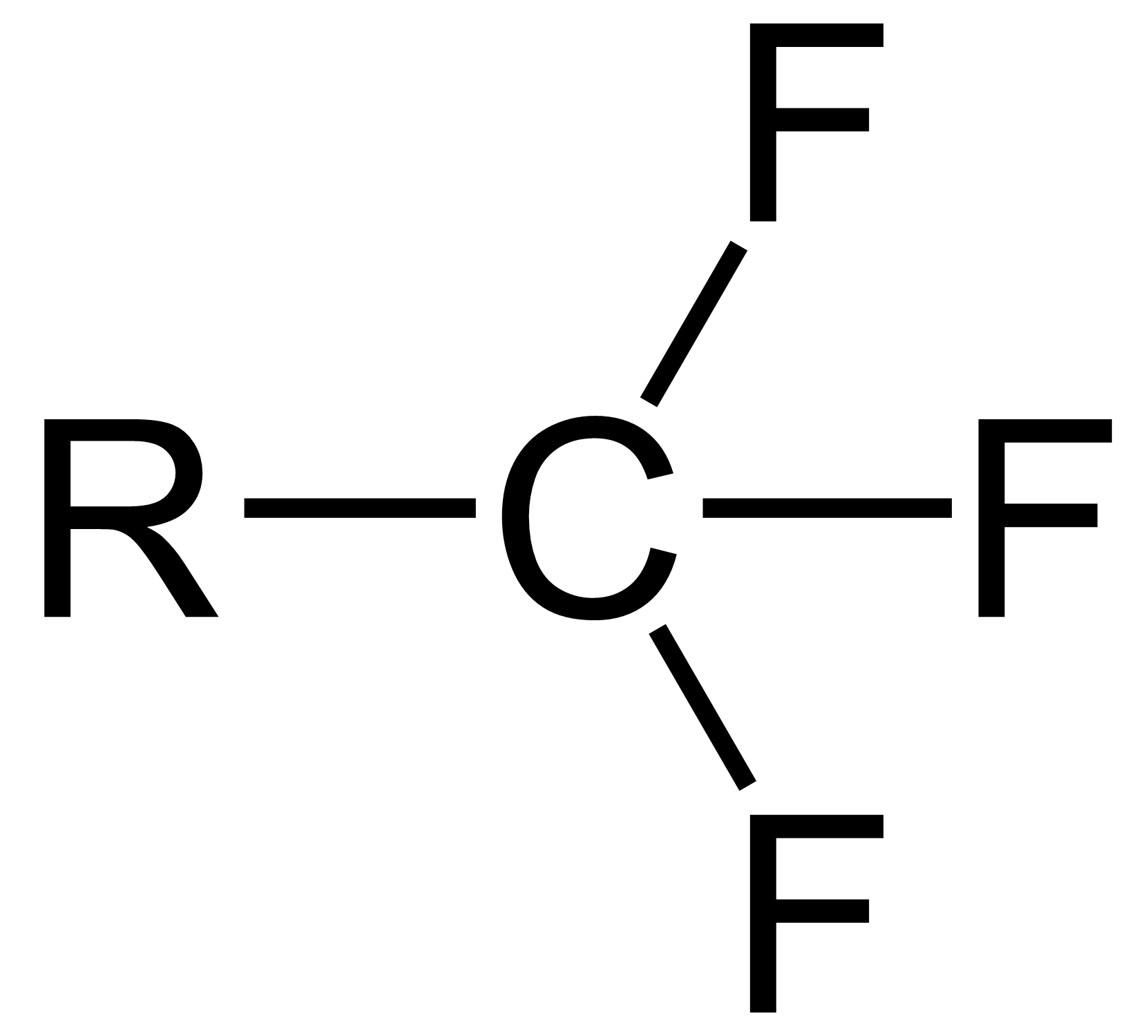
Trifluoromethyl Compounds
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone –CO–. Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Medicinal And Pharmaceutical Chemistry
A journal, from the Old French ''journal'' (meaning "daily"), may refer to: *Bullet journal, a method of personal organization *Diary, a record of what happened over the course of a day or other period *Daybook, also known as a general journal, a daily record of financial transactions *Logbook, a record of events important to the operation of a vehicle, facility, or otherwise *Record (other) *Transaction log, a chronological record of data processing *Travel journal In publishing, ''journal'' can refer to various periodicals or serials: *Academic journal, an academic or scholarly periodical **Scientific journal, an academic journal focusing on science **Medical journal, an academic journal focusing on medicine **Law review, a professional journal focusing on legal interpretation *Magazine, non-academic or scholarly periodicals in general **Trade magazine, a magazine of interest to those of a particular profession or trade **Literary magazine, a magazine devoted to literat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoromethyl Copper
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluoromethane H–, 1,1,1-trifluoroethane –, and hexafluoroacetone –CO–. Compounds with this group are a subclass of the organofluorines. Properties The trifluoromethyl group has a significant electronegativity that is often described as being intermediate between the electronegativities of fluorine and chlorine. For this reason, trifluoromethyl-substituted compounds are often strong acids, such as trifluoromethanesulfonic acid and trifluoroacetic acid. Conversely, the trifluoromethyl group lowers the basicity of compounds like trifluoroethanol. Uses The trifluoromethyl group occurs in certain pharmaceuticals, drugs, and abiotically synthesized natural fluorocarbon based compounds. The medicinal use of the trifloromethyl group dates from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Swarts Reaction
Swarts fluorination is a process whereby the chlorine atoms in a compound – generally an organic compound, but experiments have been performed using silanes – are replaced with fluorine, by treatment with antimony trifluoride in the presence of chlorine or of antimony pentachloride. Some metal fluorides are particularly more useful than others, including silver(I) fluoride, mercurous fluoride, cobalt(II) fluoride and aforementioned antimony. Heating the mixture of the metal fluoride and the haloalkane (chlorine and bromine are replaced readily) yields the desired fluoro-alkane. In some particularly reactive cases, heating is unnecessary; shaking or stirring the reaction mixture is sufficient. This reaction has a good yield. The active species is antimony trifluorodichloride, which is produced in situ ''In situ'' (; often not italicized in English) is a Latin phrase that translates literally to "on site" or "in position." It can mean "locally", "on site", "on the premise ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimony Pentachloride
Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to dissolved chlorine. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive substance and must be stored in glass or PTFE containers. Preparation and structure Antimony pentachloride is prepared by passing chlorine gas into molten antimony trichloride: :SbCl3 + Cl2 → SbCl5 Gaseous SbCl5 has a trigonal bipyramidal structure. Reactions This compounds reacts with water to form antimony pentoxide and hydrochloric acid: :2 SbCl5 + 5 H2O → Sb2O5 + 10 HCl The mono- and tetrahydrates are known, SbCl5·H2O and SbCl5·4H2O. This compound forms adducts with many Lewis bases. SbCl5 is a soft Lewis acid and its ECW model parameters are EA = 3.64 and CA = 10.42. It is used as the standard Lewis acid in the Gutmann scale of Lewis basicity. It is also a strong oxidizing agent. For example aromatic ethers are oxidized to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimony Trifluoride
Antimony trifluoride is the inorganic compound with the formula SbF3. Sometimes called Swarts' reagent, is one of two principal fluorides of antimony, the other being SbF5. It appears as a white solid. As well as some industrial applications, it is used as a reagent in inorganic and organofluorine chemistry. Preparation and structure In solid SbF3, the Sb centres have octahedral molecular geometry and are linked by bridging fluoride ligands. Three Sb–F bonds are short (192 pm) and three are long (261 pm). Because it is a polymer, SbF3 is far less volatile than related compounds AsF3 and SbCl3. SbF3 is prepared by treating antimony trioxide with hydrogen fluoride: :Sb2O3 + 6 HF → 2 SbF3 + 3 H2O The compound is a mild Lewis acid, hydrolyzing slowly in water. With fluorine, it is oxidized to give antimony pentafluoride. :SbF3 + F2 → SbF5 Applications It is used as a fluorination reagent in organic chemistry. This application was reported by the B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur Tetrafluoride
Sulfur tetrafluoride is the chemical compound with the formula S F4. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture. Despite these unwelcome characteristics, this compound is a useful reagent for the preparation of organofluorine compounds, some of which are important in the pharmaceutical and specialty chemical industries. Structure Sulfur in SF4 is in the formal +4 oxidation state. Of sulfur's total of six valence electrons, two form a lone pair. The structure of SF4 can therefore be anticipated using the principles of VSEPR theory: it is a see-saw shape, with S at the center. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons. Consequently, the molecule has two distinct types of F ligands, two axial and two equatorial. The relevant bond distances are = 164.3 pm and = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. In c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylic Acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Examples and nomenclature Carboxylic acids are commonly identified by their trivial names. They at oftentimes have the suffix ''-ic acid''. IUPAC-recommended names also exist; in this system, carboxylic acids have an ''-oic acid'' suffix. For example, butyric acid (C3H7CO2H) is butanoic acid by IUPAC guidelines. For nomenclature of complex molecules containing a carboxylic acid, the carboxyl can be considered position one of the parent chain even if there are other substituents, such as 3-chloropropanoic acid. Alternately, it can be named as a "carboxy" or "carboxylic acid" substituent on another ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfoxaflor
Sulfoxaflor, also marketed as ''Isoclast'', is a systemic insecticide that acts as an insect neurotoxin. A pyridine and a trifluoromethyl compound, it is a member of a class of chemicals called sulfoximines, which act on the central nervous system of insects. Mechanism of action Sulfoxaflor is a systemic insecticide, acts as a neurotoxin to affected insects, and kills through contact or ingestion. Sulfoxaflor is classified for use against sap-feeding insects as a sulfoximine, which is a sub-group of insecticides that act as nicotinic acetylcholine receptor (nAChR) competitive modulators. Sulfoxaflor binds to nAChRs in place of acetylcholine. Sulfoxaflor binding causes uncontrolled nerve impulses resulting in muscle tremors followed by paralysis and death. Other nAChR competitive modulator sub-groups that bind differently on the receptor than sulfoximines include neonicotinoids, nicotine, and butenolides. Because sulfoxaflor binds much more strongly to insect neuron receptors tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonsteroidal Anti-inflammatory Drug
Non-steroidal anti-inflammatory drugs (NSAID) are members of a therapeutic drug class which reduces pain, decreases inflammation, decreases fever, and prevents blood clots. Side effects depend on the specific drug, its dose and duration of use, but largely include an increased risk of gastrointestinal ulcers and bleeds, heart attack, and kidney disease. The term ''non-steroidal'', common from around 1960, distinguishes these drugs from corticosteroids, which during the 1950s had acquired a bad reputation due to overuse and side-effect problems after their initial introduction in 1948. NSAIDs work by inhibiting the activity of cyclooxygenase enzymes (the COX-1 and COX-2 isoenzymes). In cells, these enzymes are involved in the synthesis of key biological mediators, namely prostaglandins, which are involved in inflammation, and thromboxanes, which are involved in blood clotting. There are two general types of NSAIDs available: non-selective, and COX-2 selective. Most N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Celecoxib
Celecoxib, sold under the brand name Celebrex among others, is a COX-2 inhibitor and nonsteroidal anti-inflammatory drug (NSAID). It is used to treat the pain and inflammation in osteoarthritis, acute pain in adults, rheumatoid arthritis, ankylosing spondylitis, painful menstruation, and juvenile rheumatoid arthritis. It may also be used to decrease the risk of colorectal adenomas in people with familial adenomatous polyposis. It is taken by mouth. Benefits are typically seen within an hour. Common side effects include abdominal pain, nausea, and diarrhea. Serious side effects may include heart attacks, strokes, gastrointestinal perforation, gastrointestinal bleeding, kidney failure, and anaphylaxis. Use is not recommended in people at high risk for heart disease. The risks are similar to other NSAIDs, such as ibuprofen and naproxen. Use in the later part of pregnancy or during breastfeeding is not recommended. Celecoxib was patented in 1993 and came into medical use in 1999 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoxetine
Fluoxetine, sold under the brand names Prozac and Sarafem, among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used for the treatment of major depressive disorder, obsessive–compulsive disorder (OCD), bulimia nervosa, panic disorder, and premenstrual dysphoric disorder. It is also approved for treatment of major depressive disorder in adolescents and children 8 years of age and over. It has also been used to treat premature ejaculation. Fluoxetine is taken by mouth. Common side effects include indigestion, trouble sleeping, sexual dysfunction, loss of appetite, dry mouth, and rash. Serious side effects include serotonin syndrome, mania, seizures, an increased risk of suicidal behavior in people under 25 years old, and an increased risk of bleeding. Antidepressant discontinuation syndrome is less likely to occur with fluoxetine than with other antidepressants, but it still happens in many cases. Fluoxetine taken during pregnan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |