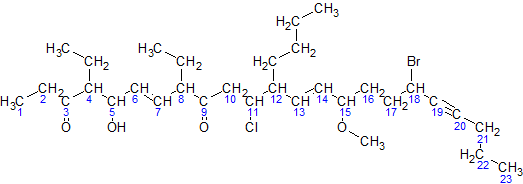
|
Thio-
The prefix thio-, when applied to a chemical, such as an ion, means that an oxygen atom in the compound has been replaced by a sulfur atom. This term is often used in organic chemistry. For example, from the word ''ether,'' referring to an oxygen-containing compound having the general chemical structure , where R and R′ are organic functional groups and O is an oxygen atom, comes the word ''thioether'', which refers to an analogous compound with the general structure , where S is a sulfur atom covalently bonded to two organic groups. A chemical reaction involving the replacement of oxygen to sulfur is called thionation or thiation. Thio- can be prefixed with ''di-'' and ''tri-'' in chemical nomenclature. The word derives (which occurs in Greek epic poetry as grc, θέ(ϝ)ειον, théweion, label=none and may come from the same root as Latin (Indo-European ''dh-w'') and may have originally meant "fumigation substance".) Examples * Thioamide * Thiocyanate * Thioether * Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Today, almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere The greatest commercial use of the element is the production o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiosulfate
Thiosulfate ( IUPAC-recommended spelling; sometimes thiosulphate in British English) is an oxyanion of sulfur with the chemical formula . Thiosulfate also refers to the compounds containing this anion, which are the salts of thiosulfuric acid, e.g. sodium thiosulfate . Thiosulfate also refers to the esters of thiosulfuric acid, e.g. ''O'',''S''-dimethyl thiosulfate . The prefix thio- indicates that the thiosulfate is a sulfate with one oxygen replaced by sulfur. Thiosulfate is tetrahedral at the central S atom. Thiosulfate salts occur naturally. Thiosulfate ion has C3v symmetry, and is produced by certain biochemical processes. It rapidly dechlorinates water and is notable for its use to halt bleaching in the paper-making industry. Thiosulfate salts are mainly used in dying in textiles and the bleaching of natural substances. Sodium thiosulfate, commonly called ''hypo'' (from "hyposulfite"), was widely used in photography to fix black and white negatives and prints after the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiourea
Thiourea () is an organosulfur compound with the formula and the structure . It is structurally similar to urea (), except that the oxygen atom is replaced by a sulfur atom (as implied by the ''thio-'' prefix); however, the properties of urea and thiourea differ significantly. Thiourea is a reagent in organic synthesis. "Thioureas" refer to a broad class of compounds with the general structure . Thioureas are related to thioamides, e.g. , where R is methyl, ethyl, etc. Structure and bonding Thiourea is a planar molecule. The C=S bond distance is 1.71 Å. The C-N distances average 1.33 Å. The weakening of the C-S bond by C-N pi-bonding is indicated by the short C=S bond in thiobenzophenone, which is 1.63 Å. Thiourea occurs in two tautomeric forms, of which the thione form predominates in aqueous solutions. The equilibrium constant has been calculated as ''K''eq is . The thiol form, which is also known as an isothiourea, can be encountered in substituted compounds such as i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate group () bonds very strong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioketone
In organic chemistry, thioketones (; also known as thiones or thiocarbonyls) are organosulfur compounds related to conventional ketones in which the oxygen has been replaced by a sulfur. Instead of a structure of , thioketones have the structure , which is reflected by the prefix "thio-" in the name of the functional group. Unhindered alkylthioketones typically tend to form polymers or rings. Structure and bonding The C=S bond length of thiobenzophenone is 1.63 Å, which is comparable to 1.64 Å, the C=S bond length of thioformaldehyde, measured in the gas phase. Due to steric interactions, the phenyl groups are not coplanar and the dihedral angle SC-CC is 36°. Unhindered dialkylthiones polymerize or oligomerize but thiocamphor is well characterized red solid. Consistent with the double bond rule, most alkyl thioketones are unstable with respect to dimerization.Organosulfur Chemistry I: Topics in Current Chemistry, 1999, Volume 204/1999, 127-181, The energy difference between ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prefix (linguistics)
A prefix is an affix which is placed before the stem of a word. Adding it to the beginning of one word changes it into another word. For example, when the prefix ''un-'' is added to the word ''happy'', it creates the word ''unhappy''. Particularly in the study of languages, a prefix is also called a preformative, because it alters the form of the words to which it is affixed. Prefixes, like other affixes, can be either inflectional, creating a new form of the word with the same basic meaning and same lexical category (but playing a different role in the sentence), or derivational, creating a new word with a new semantic meaning and sometimes also a different lexical category. Prefixes, like all other affixes, are usually bound morphemes. In English, there are no inflectional prefixes; English uses suffixes instead for that purpose. The word ''prefix'' is itself made up of the stem ''fix'' (meaning "attach", in this case), and the prefix ''pre-'' (meaning "before"), both of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioamide
A thioamide (rarely, thionamide, but also known as thiourylenes) is a functional group with the general structure R–CS–NR′R″, where R, R′, and R″ are organic groups. They are analogous to amides but they exhibit greater multiple bond character along the C-N bond, resulting in a larger rotational barrier. One of the best-known thioamides is thioacetamide, which is used as a source of the sulfide ion and is a building block in heterocyclic chemistry. Thioamides or anti-thyroid drugs are also a class of drugs that are used to control thyrotoxicosis. Preparation and structure Thioamides are typically prepared by treating amides with phosphorus sulfides such as phosphorus pentasulfide, a reaction first described in the 1870s. Alternative routes include the use of Lawesson's reagent or the reaction of nitriles with hydrogen sulfide: The Willgerodt-Kindler reaction also affords benzylthioamides. The C2NH2S core of thioamides is planar. Using thioacetamide as represent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC Nomenclature Of Organic Chemistry
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the ''Nomenclature of Organic Chemistry'' (informally called the Blue Book). Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry. To avoid long and tedious names in normal communication, the official IUPAC naming recommendations are not always followed in practice, except when it is necessary to give an unambiguous and absolute definition to a compound. IUPAC names can sometimes be simpler than older names, as with ethanol, instead of ethyl alcohol. For relatively simple molecules they can be more easily understood than non-systematic names, which must be learnt or looked over. However, the common or trivial name is often substantially ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosulfur Compounds
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, Desulfurization, the removal of which is a Claus process, major focus of oil refineries. Sulfur shares the chalcogen group with oxygen, selenium, and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium, and carbon–tellurium compounds. A classical chemical test for the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring. Isolation and occurrence Thiophene was discovered as a contaminant in benzene. It was observed that isatin (an indole) forms a blue dye if it is mixed with sulfuric acid and crude benzene. The formation of the blue indophenin had long been believed to be a reaction of benzene itself. Viktor Meyer was able to isolate thiophene as the actual substance responsible for this reaction. Thiophene and especially its derivatives occur in petroleum, sometimes in concentrations up to 1–3%. The thiophenic content of oil and coal is removed via the hydrodesulfurization (HDS) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioether
In organic chemistry, an organic sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application. Nomenclature Sulfides are sometimes called thioethers, especially in the old literature. The two organic substituents are indicated by the prefixes. (CH3)2S is called dimethylsulfide. Some sulfides are named by modifying the common name for the corresponding ether. For example, C6H5SCH3 is methyl phenyl sulfide, but is more commonly called thioanisole, since its structure is related to that for anisole, C6H5OCH3. The modern sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiocyanate
Thiocyanate (also known as rhodanide) is the anion . It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) thiocyanate was formerly used in pyrotechnics. Thiocyanate is analogous to the cyanate ion, , wherein oxygen is replaced by sulfur. is one of the pseudohalides, due to the similarity of its reactions to that of halide ions. Thiocyanate used to be known as rhodanide (from a Greek word for rose) because of the red colour of its complexes with iron. Thiocyanate is produced by the reaction of elemental sulfur or thiosulfate with cyanide: : 8 CN- + S8 -> 8 SCN- : CN- + S2O3^2- -> SCN- + SO3^2- The second reaction is catalyzed by thiosulfate sulfurtransferase, a hepatic mitochondrial enzyme, and by other sulfur transferases, which together are responsible for around 80% of cyanide metabolism in the body. Biological chemistry of thiocyanate in medicine Thiocyanate is known to be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |