|
Thiocyanate
Thiocyanate (also known as rhodanide) is the anion . It is the conjugate base of thiocyanic acid. Common derivatives include the colourless salts potassium thiocyanate and sodium thiocyanate. Mercury(II) thiocyanate was formerly used in pyrotechnics. Thiocyanate is analogous to the cyanate ion, , wherein oxygen is replaced by sulfur. is one of the pseudohalides, due to the similarity of its reactions to that of halide ions. Thiocyanate used to be known as rhodanide (from a Greek word for rose) because of the red colour of its complexes with iron. Thiocyanate is produced by the reaction of elemental sulfur or thiosulfate with cyanide: : 8 CN- + S8 -> 8 SCN- : CN- + S2O3^2- -> SCN- + SO3^2- The second reaction is catalyzed by thiosulfate sulfurtransferase, a hepatic mitochondrial enzyme, and by other sulfur transferases, which together are responsible for around 80% of cyanide metabolism in the body. Biological chemistry of thiocyanate in medicine Thiocyanate is known to b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
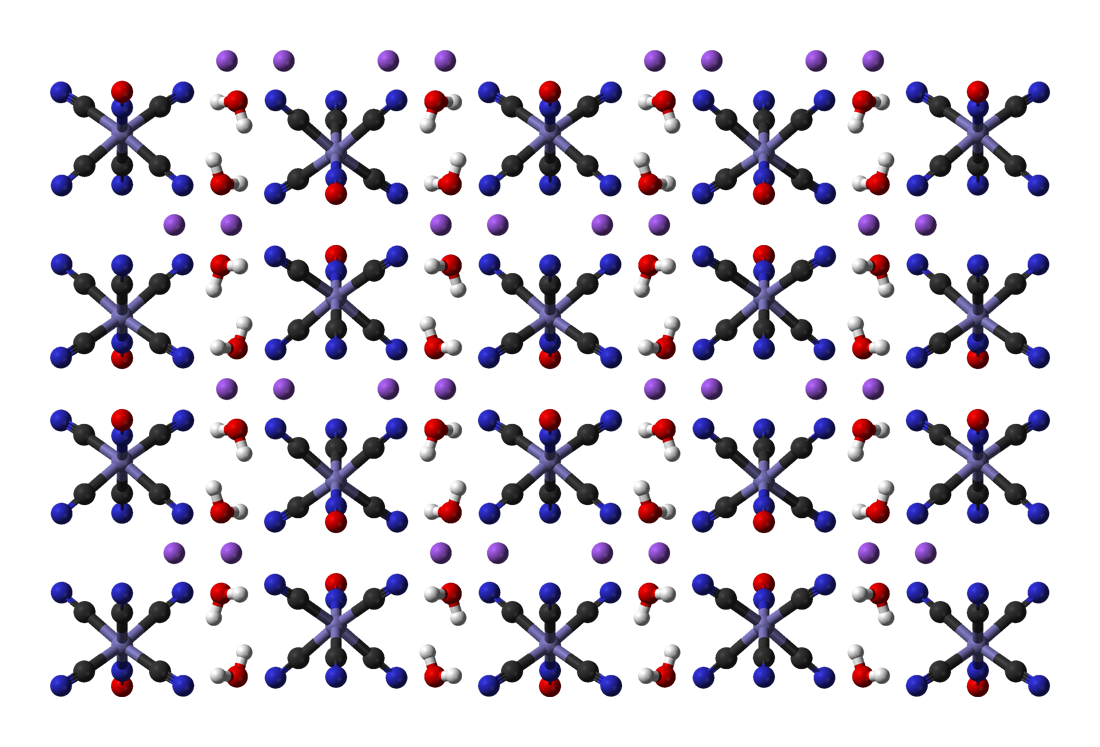
Sodium Thiocyanate
Sodium thiocyanate (sometimes called sodium sulphocyanide) is the chemical compound with the formula NaSCN. This colorless deliquescent salt is one of the main sources of the thiocyanate anion. As such, it is used as a precursor for the synthesis of pharmaceuticals and other specialty chemicals. Thiocyanate salts are typically prepared by the reaction of cyanide with elemental sulfur: :8 NaCN + S8 → 8 NaSCN Sodium thiocyanate crystallizes in an orthorhombic cell. Each Na+ center is surrounded by three sulfur and three nitrogen ligands provided by the triatomic thiocyanate anion. It is commonly used in the laboratory as a test for the presence of Fe3+ ions. Applications in chemical synthesis Sodium thiocyanate is employed to convert alkyl halides into the corresponding alkylthiocyanates. Closely related reagents include ammonium thiocyanate and potassium thiocyanate, which has twice the solubility in water. Silver thiocyanate may be used as well; the preci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(II) Thiocyanate
Mercury(II) thiocyanate (Hg(SCN)2) is an inorganic chemical compound, the coordination complex of Hg2+ and the thiocyanate anion. It is a white powder. It will produce a large, winding "snake" when ignited, an effect known as the Pharaoh's serpent. Synthesis and structure The first synthesis of mercury thiocyanate was probably completed in 1821 by Jöns Jacob Berzelius: :HgO + 2 HSCN → Hg(SCN)2 + H2O Evidence for the first pure sample was presented in 1866 prepared by a chemist named Otto Hermes. It is prepared by treating solutions containing mercury(II) and thiocyanate ions. The low solubility product of mercury thiocyanate causes it to precipitate from the solution. Most syntheses are achieved by precipitation: :Hg(NO3)2 + 2 KSCN → Hg(SCN)2 + 2KNO3 The compound adopts a polymeric structure with Hg2+ centres linearly coordinated to two S atoms with a distance of 2.381 Å. Four weak Hg2+--N interactions are indicated with distances of 2.81 Å. Uses Mercury thiocyanat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactoperoxidase
Lactoperoxidase is a peroxidase enzyme secreted from mammary, salivary and other mucosal glands including the lungs, bronchii and nose that functions as a natural and the first line of defense against bacteria and viruses. Lactoperoxidase is a member of the heme peroxidase family of enzymes. In humans, lactoperoxidase is encoded by the ''LPO'' gene. Lactoperoxidase catalyzes the oxidation of several inorganic and organic substrates by hydrogen peroxide. These substrates include bromide and iodide and therefore lactoperoxidase can be categorised as a haloperoxidase. An other important substrate is thiocyanate. The oxidized products produced through the action of this enzyme have potent and non-specific bactericidal and antiviral activities, including destruction of the influenza virus. Lactoperoxidase together with its inorganic ion substrates, hydrogen peroxide, and oxidized products is known as the lactoperoxidase system. Hence LPO is considered a very important defense agai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Thiocyanate
Potassium thiocyanate is the chemical compound with the molecular formula KSCN. It is an important salt of the thiocyanate anion, one of the pseudohalides. The compound has a low melting point relative to most other inorganic salts. Use in chemical synthesis Aqueous KSCN reacts almost quantitatively with Pb(NO3)2 to give Pb(SCN)2, which has been used to convert acyl chlorides to isothiocyanates. KSCN converts ethylene carbonate to ethylenesulfide. For this purpose, the KSCN is first melted under vacuum to remove water. In a related reaction, KSCN converts cyclohexene oxide to the corresponding episulfide. :C6H10O + KSCN → C6H10S + KOCN KSCN is also the starting product for the synthesis of carbonyl sulfide. Other uses Dilute aqueous KSCN is occasionally used for moderately realistic blood effects in film and theater. It can be painted onto a surface or kept as a colorless solution. When in contact with ferric chloride solution (or other solutions containing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pseudohalogen
Pseudohalogens are polyatomic analogues of halogens, whose chemistry, resembling that of the true halogens, allows them to substitute for halogens in several classes of chemical compounds. Pseudohalogens occur in pseudohalogen molecules, inorganic molecules of the general forms ''Ps''–''Ps'' or ''Ps''–X (where ''Ps'' is a pseudohalogen group), such as cyanogen; pseudohalide anions, such as cyanide ion; inorganic acids, such as hydrogen cyanide; as ligands in coordination complexes, such as ferouscyanide; and as functional groups in organic molecules, such as the nitrile group. Well-known pseudohalogen functional groups include cyanide, cyanate, thiocyanate, and azide. Common pseudohalogens and their nomenclature Many pseudohalogens are known by specialized common names according to where they occur in a compound. Well-known ones include (the true halogen chlorine is listed for comparison): Au− is considered to be a pseudohalogen ion due to its disproportionation react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hypothiocyanite
Hypothiocyanite is the anion SCNsup>− and the conjugate base of hypothiocyanous acid (HOSCN). It is an organic compound part of the thiocyanates as it contains the functional group SCN. It is formed when an oxygen is singly bonded to the thiocyanate group. Hypothiocyanous acid is a fairly weak acid; its acid dissociation constant (p''K''a) is 5.3. Hypothiocyanite is formed by peroxidase catalysis of hydrogen peroxide and thiocyanate: : H2O2 + SCN− → OSCN− + H2O As a bactericide Hypothiocyanite occurs naturally in the antimicrobial immune system of the human respiratory tract in a redox reaction catalyzed by the enzyme lactoperoxidase. It has been researched extensively for its capabilities as an alternative antibiotic as it is harmless to human body cells while being cytotoxic to bacteria. The exact processes for making hypothiocyanite have been patented as such an effective antimicrobial has many commercial applications. Mechanism of action Lactoperoxidase-catalyse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Nitroprusside
Sodium nitroprusside (SNP), sold under the brand name Nitropress among others, is a medication used to lower blood pressure. This may be done if the blood pressure is very high and resulting in symptoms, in certain types of heart failure, and during surgery to decrease bleeding. It is used by continuous injection into a vein. Onset is nearly immediate and effects last for up to ten minutes. It is available as a generic medication. Side effects and mechanism Common side effects include low blood pressure and cyanide toxicity. Other serious side effects include methemoglobinemia. It is not generally recommended during pregnancy due to concerns of side effects. High doses are not recommended for more than ten minutes. It works by increasing nitric oxide levels in the blood, which increases cGMP levels in cells, and causes dilation of blood vessels. History, society and culture Sodium nitroprusside was discovered as early as 1850 and found to be useful in medicine in 1928. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cystic Fibrosis
Cystic fibrosis (CF) is a rare genetic disorder that affects mostly the lungs, but also the pancreas, liver, kidneys, and intestine. Long-term issues include difficulty breathing and coughing up mucus as a result of frequent lung infections. Other signs and symptoms may include sinus infections, poor growth, fatty stool, clubbing of the fingers and toes, and infertility in most males. Different people may have different degrees of symptoms. Cystic fibrosis is inherited in an autosomal recessive manner. It is caused by the presence of mutations in both copies of the gene for the cystic fibrosis transmembrane conductance regulator (CFTR) protein. Those with a single working copy are carriers and otherwise mostly healthy. CFTR is involved in the production of sweat, digestive fluids, and mucus. When the CFTR is not functional, secretions which are usually thin instead become thick. The condition is diagnosed by a sweat test and genetic testing. Screening of infants at bi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiosulfate Sulfurtransferase
Rhodanese, also known as rhodanase, thiosulfate sulfurtransferase, thiosulfate cyanide transsulfurase, and thiosulfate thiotransferase, at the International Union of Biochemistry and Molecular Biology is a that detoxifies (CN−) by converting it to (SCN−). This reaction takes place in two steps. The di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiocyanic Acid
Thiocyanic acid is a chemical compound with the formula HSCN and structure , which exists as a tautomer with isothiocyanic acid (HNCS). The iso- form tends to dominate with the material being about 95% isothiocyanic acid in the vapor phase. : It is a moderately strong acid, with a p''K''a of 1.1 at 20 °C and extrapolated to zero ionic strength. HSCN is predicted to have a triple bond between carbon and nitrogen. It has been observed spectroscopically but has not been isolated as a pure substance. The salts and ester In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides ...s of thiocyanic acid are known as thiocyanates. The salts are composed of the thiocyanate ion (−SCN) and a suitable metal cation (e.g., potassium thiocyanate, KSCN). The esters of thiocyanic acid have t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyanide
Cyanide is a naturally occurring, rapidly acting, toxic chemical that can exist in many different forms. In chemistry, a cyanide () is a chemical compound that contains a functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom. In inorganic cyanides, the cyanide group is present as the anion . Soluble salts such as sodium cyanide (NaCN) and potassium cyanide (KCN) are highly toxic. Hydrocyanic acid, also known as hydrogen cyanide, or HCN, is a highly volatile liquid that is produced on a large scale industrially. It is obtained by acidification of cyanide salts. Organic cyanides are usually called nitriles. In nitriles, the group is linked by a covalent bond to carbon. For example, in acetonitrile (), the cyanide group is bonded to methyl (). Although nitriles generally do not release cyanide ions, the cyanohydrins do and are thus rather toxic. Bonding The cyanide ion is isoelectronic with carbon m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |