|
TPOX
Partial oxidation (POX) is a type of chemical reaction. It occurs when a substoichiometric fuel-air mixture is partially combusted in a reformer, creating a hydrogen-rich syngas which can then be put to further use, for example in a fuel cell. A distinction is made between ''thermal partial oxidation'' (TPOX) and ''catalytic partial oxidation'' (CPOX). Principle Partial oxidation is a technically mature process in which natural gas or a heavy hydrocarbon fuel (heating oil) is mixed with a limited amount of oxygen in an exothermic process. * General reaction: + \frac\mathit -> \mathit + \frac\mathitH2 * Idealized reaction for heating oil: + 6O2 -> + 12H2 * Idealized reaction for coal: + 12O2 -> + 6H2 The formulas given for coal and heating oil show only a typical representative of these complex fuels. Water may be added to lower the combustion temperature and reduce soot formation. Yields are below stoichiometric due to some fuel being fully combusted to carbon dioxide and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glossary Of Fuel Cell Terms
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few. A ; Activation loss : See overpotential ; Adsorption : Adsorption is a process that occurs when a gas or liquid solute accumulates on the surface of a solid or a liquid (adsorbent), forming a film of molecules or atoms (the adsorbate). ; Alkali : In chemistry, an alkali is a basic, ionic salt of an alkali metal or alkaline earth metal element. ; Alkali anion exchange membrane : An alkali anion exchange membrane (AAEM) is a semipermeable membrane generally made from ionomers and designed to conduct anions while being impermeable to gases such as oxygen or hydrogen. ; Alkaline fuel cell : Alkaline fuel cell (AFC) also known as the Bacon fuel cell. ; Alloy : An alloy is a solid solution or homogeneous mix ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic Partial Oxidation
Partial oxidation (POX) is a type of chemical reaction. It occurs when a substoichiometric fuel-air mixture is partially combusted in a reformer, creating a hydrogen-rich syngas which can then be put to further use, for example in a fuel cell. A distinction is made between ''thermal partial oxidation'' (TPOX) and ''catalytic partial oxidation'' (CPOX). Principle Partial oxidation is a technically mature process in which natural gas or a heavy hydrocarbon fuel (heating oil) is mixed with a limited amount of oxygen in an exothermic process. * General reaction: + \frac\mathit -> \mathit + \frac\mathitH2 * Idealized reaction for heating oil: + 6O2 -> + 12H2 * Idealized reaction for coal: + 12O2 -> + 6H2 The formulas given for coal and heating oil show only a typical representative of these complex fuels. Water may be added to lower the combustion temperature and reduce soot formation. Yields are below stoichiometric due to some fuel being fully combusted to carbon dioxide and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Small Stationary Reformer
A small stationary reformer is an on-site device used for the production of hydrogen from hydrocarbons on a small scale. Types Plate-type steam methane reformers Multi-tube steam reformer Membrane reactor A membrane reactor is a device where oxygen separation, steam reforming and POX is combined in a single step. In 1997 Argonne National Laboratory and Amoco published a paper "Ceramic membrane reactor for converting methane to syngas" which resulted in different small scale systems that combined an ATR based oxygen membrane with a water-gas shift reactor and a hydrogen membrane. POX reactor Partial oxidation (POX) is a type of chemical reaction. It occurs when a substoichiometric fuel-air mixture is partially combusted in a reformer, creating a hydrogen-rich syngas which can then be put to further use, for example in a fuel cell. A distinction is made between ''thermal partial oxidation'' (TPOX) and ''catalytic partial oxidation'' (CPOX). Steam methane reformers with catalyst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
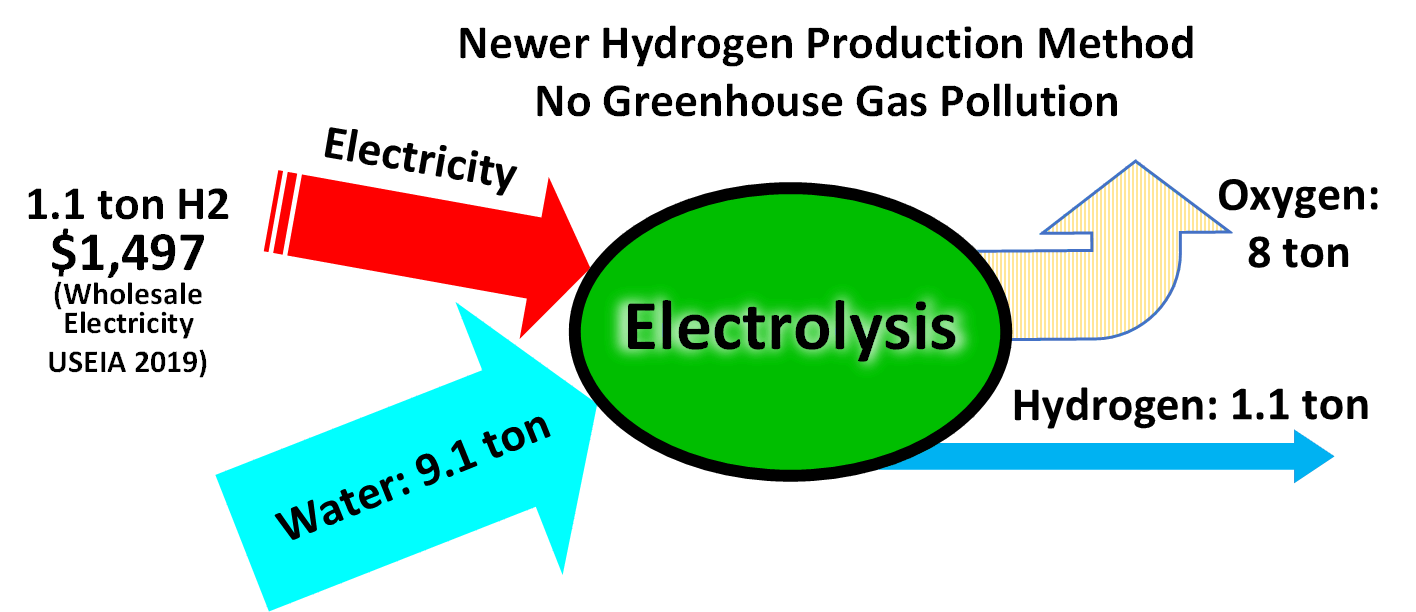
Hydrogen Production
Hydrogen gas is produced by several industrial methods. Nearly all of the world's current supply of hydrogen is created from fossil fuels. Article in press. Most hydrogen is ''gray hydrogen'' made through steam methane reforming. In this process, hydrogen is produced from a chemical reaction between steam and methane, the main component of natural gas. Producing one tonne of hydrogen through this process emits 6.6–9.3 tonnes of carbon dioxide. When carbon capture and storage is used to remove a large fraction of these emissions, the product is known as ''blue hydrogen''. ''Green hydrogen'' is usually understood to be produced from Renewable energy, renewable electricity via electrolysis of water. Less frequently, definitions of ''green hydrogen'' include hydrogen produced from other low-emission sources such as Biomass (energy), biomass. Producing green hydrogen is currently more expensive than producing gray hydrogen, and the efficiency of energy conversion is inherently low. O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, energy change as new products are generated. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with the chemical formula S8. Elemental sulfur is a bright yellow, crystalline solid at room temperature. Sulfur is the tenth most abundant element by mass in the universe and the fifth most common on Earth. Though sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in ancient times, being mentioned for its uses in ancient India, ancient Greece, China, and ancient Egypt. Historically and in literature sulfur is also called brimstone, which means "burning stone". Almost all elemental sulfur is produced as a byproduct of removing sulfur-containing contaminants from natural gas and petroleum.. Downloahere Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reactions
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an energy change as new products are generated. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Timeline Of Hydrogen Technologies
This is a timeline of the history of hydrogen technology. Timeline 16th century * c. 1520 – First recorded observation of hydrogen by Paracelsus through dissolution of metals (iron, zinc, and tin) in sulfuric acid. 17th century * 1625 – First description of hydrogen by Jan Baptist van Helmont, Johann Baptista van Helmont. First to use the word "gas". * 1650 – Theodore de Mayerne, Turquet de Mayerne obtains a gas or "inflammable air" by the action of dilute sulphuric acid on iron. * 1662 – Boyle's law (gas law relating pressure and volume). * 1670 – Robert Boyle produces hydrogen by reacting metals with acid. * 1672 – "New Experiments touching the Relation between Flame and Air" by Robert Boyle. * 1679 – Denis Papin – safety valve. * 1700 – Nicolas Lemery shows that the gas produced in the sulfuric acid/iron reaction is explosive in air. 18th century * 1755 – Joseph Black confirms that different gases exist. / Latent heat * 1766 – Henry Cavendish publishes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PROX
PROX is an acronym for PReferential OXidation, that refers to the preferential oxidation of carbon monoxide in a gas mixture by a catalyst. It is intended to remove trace amounts of CO from H2/CO/CO2 mixtures produced by steam reforming and water-gas shift. An ideal PROX catalyst preferentially oxidizes carbon monoxide (CO) using a heterogeneous catalyst placed upon a ceramic support. Catalysts include metals such as platinum, platinum/iron, platinum/ruthenium, gold nanoparticles as well as novel copper oxide/ceramic conglomerate catalysts. Motivation This reaction is a considerable subject area of research with implications for fuel cell design. Its main utility lies in the removal of carbon monoxide (CO) from the fuel cell's feed gas. CO poisons the catalyst of most low-temperature fuel cells. Carbon monoxide is often produced as a by-product from steam reforming of hydrocarbons, which produces hydrogen and CO. It is possible to consume most of the CO by reacting it with steam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Industrial Gas
Industrial gases are the gaseous materials that are Manufacturing, manufactured for use in Industrial sector, industry. The principal gases provided are nitrogen, oxygen, carbon dioxide, argon, hydrogen, helium and acetylene, although many other gases and mixtures are also available in gas cylinders. The industry producing these gases is also known as industrial gas, which is seen as also encompassing the supply of equipment and technology to produce and use the gases. Their production is a part of the wider chemical Industry (where industrial gases are often seen as "specialty chemicals"). Industrial gases are used in a wide range of industries, which include petroleum industry, oil and gas, petrochemicals, chemical industry, chemicals, electric power industry, power, mining, steelmaking, metals, Environmentalism, environmental protection, medicine, Pharmaceutical drug, pharmaceuticals, biotechnology, food Industry, food, Water industry, water, fertilizers, nuclear power, electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
University Of Illinois
The University of Illinois Urbana-Champaign (UIUC, U of I, Illinois, or University of Illinois) is a public university, public land-grant university, land-grant research university in the Champaign–Urbana metropolitan area, Illinois, United States. Established in 1867, it is the founding campus and Flagship#Colleges and universities in the United States, flagship institution of the University of Illinois System. With over 59,000 students, the University of Illinois is one of the List of United States public university campuses by enrollment, largest public universities by enrollment in the United States. The university contains 16 schools and colleges and offers more than 150 undergraduate and over 100 graduate programs of study. The university holds 651 buildings on and its annual operating budget in 2016 was over $2 billion. The University of Illinois Urbana-Champaign also operates Research Park at the University of Illinois Urbana-Champaign, a research park home to innova ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |