|
Steviol Structure
Steviol is a diterpene first isolated from the plant ''Stevia rebaudiana'' in 1931. Its chemical structure was not fully elucidated until 1960. Steviol occurs in the plant as steviol glycosides, sweet compounds that have found widespread use as sugar substitutes. The aglycon is prepared by enzymatic hydrolysis, since upon acid treatment steviol will undergo Wagner-Meerwein rearrangement to the very stable isosteviol. Biosynthesis In ''Stevia rebaudiana'', the biosynthesis of steviol is confined to green tissues. The precursors of steviol are synthesized via the non-mevalonate pathway located in plant cell plastids, which produces isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). IPP and DMAPP are converted to geranylgeranyl diphosphate (GGDP), which is the precursor of many diterpenoids, by GGDP synthase. GPDP is made into a cyclic compound, copalyl diphosphate (CDP), by CDP synthase, after which kaurene is produced by another cyclization catalyzed b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diterpene
Diterpenes are a class of chemical compounds composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. They are known to be antimicrobial and antiinflammatory. Structures As with most terpenes a huge number of potential structures exists, which may be broadly divided according to the number of rings present. Biosynthesis Diterpenes are derived from the addition of one IPP unit to FPP to form geranylgeranyl-pyrophosphate (GGPP). From GGPP, structural diversity is achieved mainly by two classes of enzymes; the diterpene synthases and cytochromes P450. Several diterpenes are produced by plants and cyanobacteria. GGPP is also the precursor for the synthesis of the phytane by the action of the enzyme geranylger ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Compound
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where all the atoms are carbon (i.e., are carbocycles), none of the atoms are carbon (inorganic cyclic compounds), or where both carbon and non-carbon atoms are present (heterocyclic compounds). Depending on the ring size, the bond order of the individual links between ring atoms, and their arrangements within the rings, carbocyclic and heterocyclic compounds may be aromatic or non-aromatic; in the latter case, they may vary from being fully saturated to having varying numbers of multiple bonds between the ring atoms. Because of the tremendous diversity allowed, in combination, by the valences of common atoms and their ability to form rings, the number of possible cyclic structures, even of small size (e.g., < 17 total atoms) numbers in the many b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diterpenes
Diterpenes are a class of chemical compounds composed of four isoprene units, often with the molecular formula C20H32. They are biosynthesized by plants, animals and fungi via the HMG-CoA reductase pathway, with geranylgeranyl pyrophosphate being a primary intermediate. Diterpenes form the basis for biologically important compounds such as retinol, retinal, and phytol. They are known to be antimicrobial and antiinflammatory. Structures As with most terpenes a huge number of potential structures exists, which may be broadly divided according to the number of rings present. Biosynthesis Diterpenes are derived from the addition of one IPP unit to FPP to form geranylgeranyl-pyrophosphate (GGPP). From GGPP, structural diversity is achieved mainly by two classes of enzymes; the diterpene synthases and cytochromes P450. Several diterpenes are produced by plants and cyanobacteria. GGPP is also the precursor for the synthesis of the phytane by the action of the enzyme geranylger ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bio Synthesis Of Steviol Glycoside 01
Bio or BIO may refer to: Computing * bio(4), a pseudo-device driver in RAID controller management interface in OpenBSD and NetBSD * Block I/O, a concept in computer data storage Politics * Julius Maada Bio (born 1964), Sierra Leonean politician, president since April 4, 2018 Media and entertainment * Bio (Australian TV channel) * The Biography Channel (UK and Ireland) * Bio (graffiti artist) Wilfredo Feliciano (born 1966) * ''Bio'' (album), a Chuck Berry album released in 1973 Organizations * Bedford Institute of Oceanography * Biographers International Organization * Biotechnology Innovation Organization * Belgian Investment Company for Developing Countries Energy * Biofuel, fuel made from biomass **Biodiesel, the biofuel alternative for diesel **Biogas, a blend of gasses formed by the breakdown of organic matter used in renewable energy production **Biogasoline, the biofuel alternative for gasoline Places * Bio, Azerbaijan, village in Astara Rayon * Bio, Lot, commune in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosylation
Glycosylation is the reaction in which a carbohydrate (or ' glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not always in chemistry), glycosylation usually refers to an enzyme-catalysed reaction, whereas glycation (also 'non-enzymatic glycation' and 'non-enzymatic glycosylation') may refer to a non-enzymatic reaction (though in practice, 'glycation' often refers more specifically to Maillard-type reactions). Glycosylation is a form of co-translational and post-translational modification. Glycans serve a variety of structural and functional roles in membrane and secreted proteins. The majority of proteins synthesized in the rough endoplasmic reticulum undergo glycosylation. Glycosylation is also present in the cytoplasm and nucleus as the ''O''-GlcNAc modification. Aglycosylation is a feature of engineered antibodies to bypass glycosylation. Five clas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NADPH
Nicotinamide adenine dinucleotide phosphate, abbreviated NADP or, in older notation, TPN (triphosphopyridine nucleotide), is a cofactor used in anabolic reactions, such as the Calvin cycle and lipid and nucleic acid syntheses, which require NADPH as a reducing agent ('hydrogen source'). It is used by all forms of cellular life. NADPH is the reduced form of NADP. NADP differs from NAD by the presence of an additional phosphate group on the 2' position of the ribose ring that carries the adenine moiety. This extra phosphate is added by NAD+ kinase and removed by NADP+ phosphatase. Biosynthesis NADP In general, NADP+ is synthesized before NADPH is. Such a reaction usually starts with NAD+ from either the de-novo or the salvage pathway, with NAD+ kinase adding the extra phosphate group. ADP-ribosyl cyclase allows for synthesis from nicotinamide in the salvage pathway, and NADP+ phosphatase can convert NADPH back to NADH to maintain a balance. Some forms of the NAD+ kinas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ent-kaurenoic Acid Oxidase
Ent-kaurenoic acid oxidase () is an enzyme with systematic name ''ent-kaur-16-en-19-oate,NADPH:oxygen oxidoreductase (hydroxylating)''. This enzyme catalyses the following chemical reaction : ent-kaur-16-en-19-oate + 3 NADPH + 3 H+ + 3 O2 \rightleftharpoons gibberellin A12 + 3 NADP+ + 4 H2O (overall reaction) :(1a) ent-kaur-16-en-19-oate + NADPH + H+ + O2 \rightleftharpoons ent-7alpha-hydroxykaur-16-en-19-oate + NADP+ + H2O :(1b) ent-7alpha-hydroxykaur-16-en-19-oate + NADPH + H+ + O2 \rightleftharpoons gibberellin A12 aldehyde + NADP+ + 2 H2O :(1c) gibberellin A12 aldehyde + NADPH + H+ + O2 \rightleftharpoons gibberellin A12 + NADP+ + H2O Ent-kaurenoic acid oxidase requires cytochrome P450 Cytochromes P450 (CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that functions as monooxygenases. In mammals, these proteins oxidize steroids, fatty acids, and xenobiotics, and are .... References External links * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kaurenoic Acid
Kaurenoic acid (ent-kaur-16-en-19-oic acid or Kauren-19-oic acid) is a diterpene with antibacterial activity against Gram-positive bacteria. However its low solubility and blood lytic activity on erythrocytes might make it a poor pharmaceutical candidate. Kaurenoic acid also has uterine relaxant activity via calcium blockade and opening ATP-sensitive potassium channels. Kaurenoic acid is found in several plants such as Copaifera. It is a potential biomarker for the presence of sunflower in foods. Medical use Kaurenoic acid has been studied for its medicinal properties and seems to have anti-inflammatory, antiulcerogenic, antitumor, antinociceptive, antimelanoma, antitilipoperoxidation, antioxidant and antimicrobial properties. Kaurenoic acid decreases leukocyte migration. It seems to inhibit histamine and serotonin pathways, in addition to antiprotozoal activities against '' Trypanosoma cruzi'' and ''Leishmania amazonensis ''Leishmania amazonensis'' is a parasite responsibl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
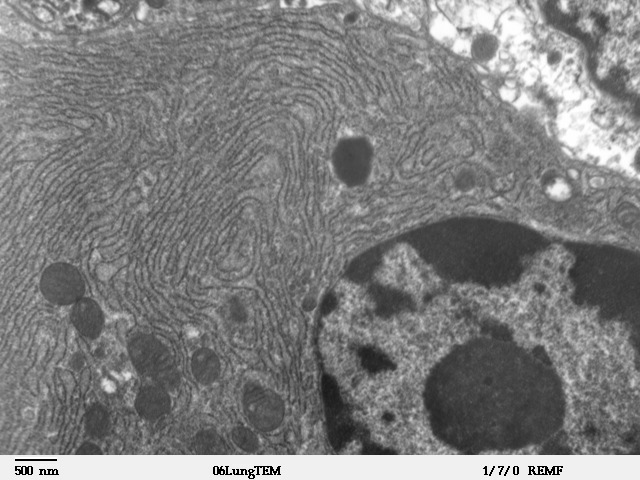
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. The two types of ER share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of cells contain different ratios of the two types of ER depending on the activities of the cell. RER is found mainly toward the nucleus of cell and SER towards the cell membrane or plasma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ent-kaurene Synthase
The enzyme ''ent''-kaurene synthase (EC 4.2.3.19) catalyzes the chemical reaction :''ent''-copalyl diphosphate \rightleftharpoons ''ent''-kaurene + diphosphate This enzyme belongs to the family of lyases, specifically those carbon-oxygen lyases acting on phosphates. The systematic name of this enzyme class is ''ent''-copalyl-diphosphate diphosphate-lyase (cyclizing, ''ent''-kaurene-forming). Other names in common use include ''ent''-kaurene synthase B, ''ent''-kaurene synthetase B, ''ent''-copalyl-diphosphate diphosphate-lyase, and (cyclizing). This enzyme participates in diterpenoid biosynthesis. In ''Stevia'' In ''Stevia'' spp., ''ent''-kaurene synthase is a required part of the biosynthesis of steviol. Hajihashemi ''et al.'', 2013 find that it is involved in the drought stress response and because it mimics drought stress paclobutrazol toxicity. Both inhbit transcription of steviol glycoside Steviol glycosides are the chemical compounds responsible for the sweet taste of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copalyl Diphosphate Synthase
In enzymology, a copalyl diphosphate synthase () is an enzyme that catalyzes the chemical reaction :geranylgeranyl diphosphate \rightleftharpoons (+)-copalyl diphosphate Hence, this enzyme has one substrate, geranylgeranyl diphosphate, and one product, (+)-copalyl diphosphate. This enzyme belongs to the family of isomerases, specifically the class of intramolecular lyases. The systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature. A semisystematic name or semitrivial ... of this enzyme class is (+)-copalyl-diphosphate lyase (decyclizing). This enzyme participates in diterpenoid biosynthesis. References * * * * * EC 5.5.1 Enzymes of unknown structure {{isomerase-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |