|
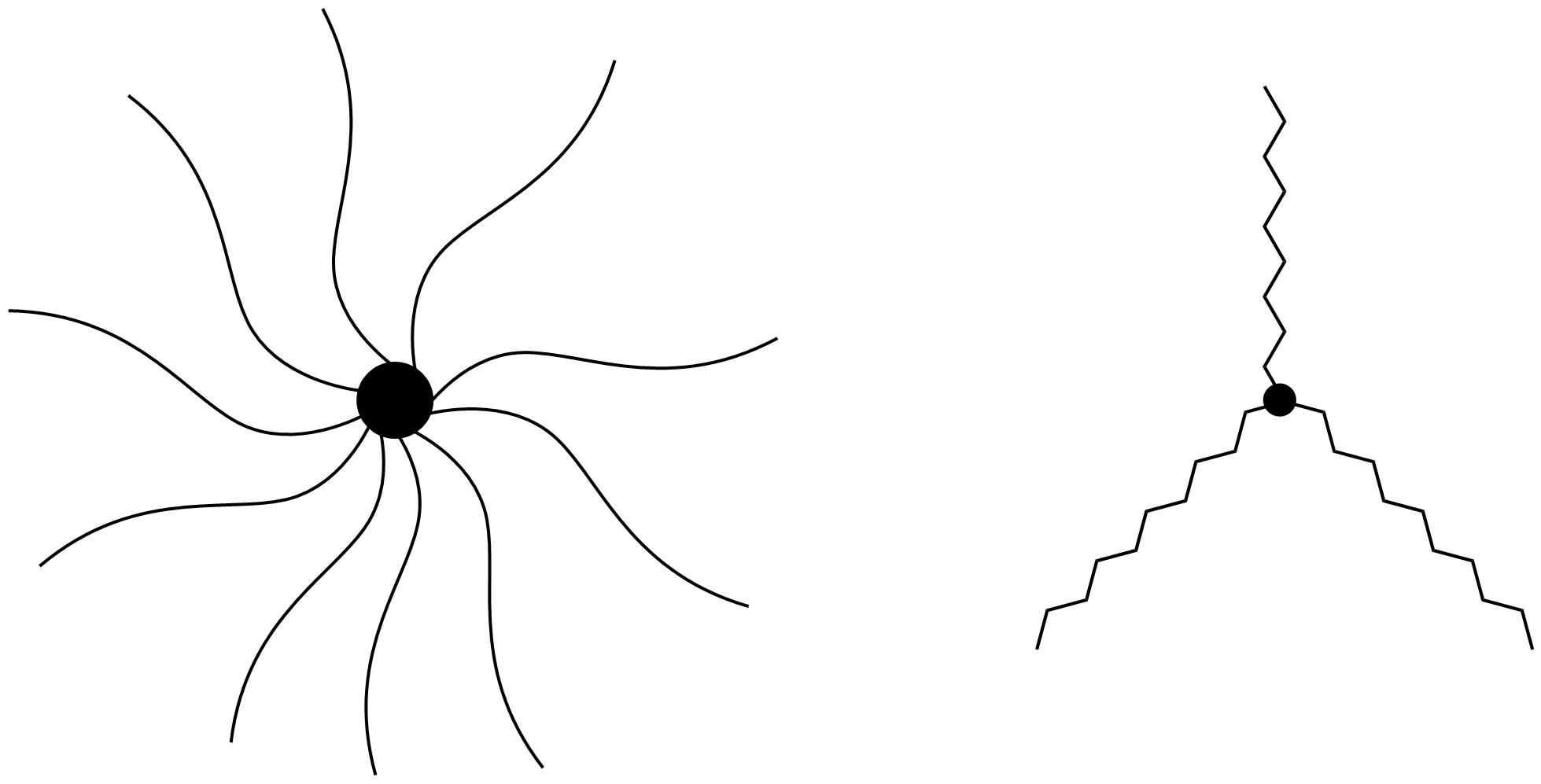
Star-shaped Polymer
Star-shaped polymers are the simplest class of branched polymers with a general structure consisting of several (at least three) linear chains connected to a central core. The core, or the center, of the polymer can be an atom, molecule, or macromolecule; the chains, or "arms", consist of variable-length organic chains. Star-shaped polymers in which the arms are all equivalent in length and structure are considered homogeneous, and ones with variable lengths and structures are considered heterogeneous. Star-shaped polymers' unique shape and associated properties, such as their compact structure, high arm density, efficient synthetic routes, and unique rheological properties make them promising tools for use in drug delivery, other biomedical applications, thermoplastics, and nanoelectronicsDrew C. Forman ; Florian Wieberger ; Andre Gröschel ; Axel H. E. Müller ; Hans-Werner Schmidt ; Christopher K. Ober; Comparison of star and linear ArF resists. Proc. SPIE 7639, Advances i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Star Shaped Polymers
A star is an astronomical object comprising a luminous spheroid of plasma (physics), plasma held together by its gravity. The List of nearest stars and brown dwarfs, nearest star to Earth is the Sun. Many other stars are visible to the naked eye at night sky, night, but their immense distances from Earth make them appear as fixed stars, fixed points of light. The most prominent stars have been categorised into constellations and asterism (astronomy), asterisms, and many of the brightest stars have proper names. Astronomers have assembled star catalogues that identify the known stars and provide standardized stellar designations. The observable universe contains an estimated to stars. Only about 4,000 of these stars are visible to the naked eye, all within the Milky Way galaxy. A star's life star formation, begins with the gravitational collapse of a gaseous nebula of material composed primarily of hydrogen, along with helium and trace amounts of heavier elements. Its stellar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Abstracts Service
CAS (formerly Chemical Abstracts Service) is a division of the American Chemical Society. It is a source of chemical information. CAS is located in Columbus, Ohio, United States. Print periodicals ''Chemical Abstracts'' is a periodical index that provides numerous tools such as SciFinder as well as tagged keywords, summaries, indexes of disclosures, and structures of compounds in recently published scientific documents. Approximately 8,000 journals, technical reports, dissertations, conference proceedings, and new books, available in at least 50 different languages, are monitored yearly, as are patent specifications from 27 countries and two international organizations. ''Chemical Abstracts'' ceased print publication on January 1, 2010. Databases The two principal databases that support the different products are CAplus and Registry. CAS References CAS References consists of bibliographic information and abstracts for all articles in chemical journals worldwide, and chemistry-r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallinity
Crystallinity refers to the degree of structural order in a solid. In a crystal, the atoms or molecules are arranged in a regular, periodic manner. The degree of crystallinity has a big influence on hardness, density, Transparency and translucency, transparency and diffusion. In an ideal gas, the relative positions of the atoms or molecules are completely random. Amorphous materials, such as liquids and glasses, represent an intermediate case, having order over short distances (a few atomic or molecular spacings) but not over longer distances. Many materials, such as glass-ceramics and some polymers, can be prepared in such a way as to produce a mixture of crystalline and Amorphous solid, amorphous regions. In such cases, crystallinity is usually specified as a percentage of the volume of the material that is crystalline. Even within materials that are completely crystalline, however, the degree of structural perfection can vary. For instance, most metallic alloys are crystalline ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallization Of Polymers
Crystallization of polymers is a process associated with partial alignment of their molecular chains. These chains fold together and form ordered regions called Lamella (materials), lamellae, which compose larger spheroidal structures named Spherulite (polymer physics), spherulites. Polymers can crystallize upon cooling from melting, mechanical stretching or solvent evaporation. Crystallization affects optical, mechanical, thermal and chemical properties of the polymer. The degree of crystallinity is estimated by different analytical methods and it typically ranges between 10 and 80%, with crystallized polymers often called "semi-crystalline". The properties of semi-crystalline polymers are determined not only by the degree of crystallinity, but also by the size and orientation of the molecular chains. Crystallization mechanisms Solidification from the melt Polymers are composed of long molecular chains which form irregular, entangled coils in the melt. Some polymers retain such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melting Point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depends on pressure and is usually specified at a standard pressure such as 1 atmosphere or 100 kPa. When considered as the temperature of the reverse change from liquid to solid, it is referred to as the freezing point or crystallization point. Because of the ability of substances to supercool, the freezing point can easily appear to be below its actual value. When the "characteristic freezing point" of a substance is determined, in fact, the actual methodology is almost always "the principle of observing the disappearance rather than the formation of ice, that is, the melting point." Examples For most substances, melting and freezing points are approximately equal. For example, the melting point ''and'' freezing point of mercury is . How ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water. Viscosity quantifies the internal frictional force between adjacent layers of fluid that are in relative motion. For instance, when a viscous fluid is forced through a tube, it flows more quickly near the tube's axis than near its walls. Experiments show that some stress (such as a pressure difference between the two ends of the tube) is needed to sustain the flow. This is because a force is required to overcome the friction between the layers of the fluid which are in relative motion. For a tube with a constant rate of flow, the strength of the compensating force is proportional to the fluid's viscosity. In general, viscosity depends on a fluid's state, such as its temperature, pressure, and rate of deformation. However, the dependence on some of these properties is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscosity
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water. Viscosity quantifies the internal frictional force between adjacent layers of fluid that are in relative motion. For instance, when a viscous fluid is forced through a tube, it flows more quickly near the tube's axis than near its walls. Experiments show that some stress (such as a pressure difference between the two ends of the tube) is needed to sustain the flow. This is because a force is required to overcome the friction between the layers of the fluid which are in relative motion. For a tube with a constant rate of flow, the strength of the compensating force is proportional to the fluid's viscosity. In general, viscosity depends on a fluid's state, such as its temperature, pressure, and rate of deformation. However, the dependence on some of these properties is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radius Of Gyration
''Radius of gyration'' or gyradius of a body about the axis of rotation is defined as the radial distance to a point which would have a moment of inertia the same as the body's actual distribution of mass, if the total mass of the body were concentrated there. Mathematically the radius of gyration is the root mean square distance of the object's parts from either its center of mass or a given axis, depending on the relevant application. It is actually the perpendicular distance from point mass to the axis of rotation. One can represent a trajectory of a moving point as a body. Then radius of gyration can be used to characterize the typical distance travelled by this point. Suppose a body consists of n particles each of mass m. Let r_1, r_2, r_3, \dots , r_n be their perpendicular distances from the axis of rotation. Then, the moment of inertia I of the body about the axis of rotation is :I = m_1 r_1^2 + m_2 r_2^2 + \cdots + m_n r_n^2 : If all the masses are the same (m), then ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrodynamic Radius
The hydrodynamic radius of a macromolecule or colloid particle is R_. The macromolecule or colloid particle is a collection of N subparticles. This is done most commonly for polymers; the subparticles would then be the units of the polymer. R_ is defined by : \frac \ \stackrel\ \frac \left\langle \sum_ \frac \right\rangle where r_ is the distance between subparticles i and j, and where the angular brackets \langle \ldots \rangle represent an ensemble average. The theoretical hydrodynamic radius R_ was originally an estimate by John Gamble Kirkwood of the Stokes radius of a polymer, and some sources still use ''hydrodynamic radius'' as a synonym for the Stokes radius. Note that in biophysics, hydrodynamic radius refers to the Stokes radius, or commonly to the apparent Stokes radius obtained from size exclusion chromatography. The theoretical hydrodynamic radius R_ arises in the study of the dynamic properties of polymers moving in a solvent. It is often similar in magnitu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not consi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dynamic Mechanical Analysis
Dynamic mechanical analysis (abbreviated DMA) is a technique used to study and characterize materials. It is most useful for studying the viscoelastic behavior of polymers A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic an .... A sinusoidal stress is applied and the strain in the material is measured, allowing one to determine the complex modulus. The temperature of the sample or the frequency of the stress are often varied, leading to variations in the complex modulus; this approach can be used to locate the glass transition temperature of the material, as well as to identify transitions corresponding to other molecular motions. Theory Viscoelastic properties of materials Polymers composed of long molecular chains have unique viscoelastic properties, which combine the characteristics ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rheology
Rheology (; ) is the study of the flow of matter, primarily in a fluid ( liquid or gas) state, but also as "soft solids" or solids under conditions in which they respond with plastic flow rather than deforming elastically in response to an applied force. Rheology is a branch of physics, and it is the science that deals with the deformation and flow of materials, both solids and liquids.W. R. Schowalter (1978) Mechanics of Non-Newtonian Fluids Pergamon The term ''rheology'' was coined by Eugene C. Bingham, a professor at Lafayette College, in 1920, from a suggestion by a colleague, Markus Reiner.The Deborah Number The term was inspired by the of |