|
Squamous Cell Lung Carcinoma
Squamous-cell carcinoma (SCC) of the lung is a histologic type of non-small-cell lung carcinoma (NSCLC). It is the second most prevalent type of lung cancer after lung adenocarcinoma and it originates in the bronchi. Its tumor cells are characterized by a squamous appearance, similar to the one observed in epidermal cells. Squamous-cell carcinoma of the lung is strongly associated with tobacco smoking, more than any other forms of NSCLC. Signs and symptoms Squamous-cell lung carcinoma share most of the signs and symptoms with other forms of lung cancer. These include worsening cough, including hemoptysis, chest pain, shortness of breath and weight loss. Symptoms may result from local invasion or compression of adjacent thoracic structures such as compression involving the esophagus causing dysphagia, compression involving the laryngeal nerves causing change in voice, or compression involving the superior vena cava causing facial edema. Distant metastases may also cause pain and s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-small-cell Lung Carcinoma
Non-small-cell lung cancer (NSCLC) is any type of epithelial lung cancer other than small-cell lung carcinoma (SCLC). NSCLC accounts for about 85% of all lung cancers. As a class, NSCLCs are relatively insensitive to chemotherapy, compared to small-cell carcinoma. When possible, they are primarily treated by surgical resection with curative intent, although chemotherapy has been used increasingly both preoperatively (Neoadjuvant therapy, neoadjuvant chemotherapy) and postoperatively (Adjuvant therapy, adjuvant chemotherapy). Types The most common types of NSCLC are squamous-cell carcinoma, large-cell carcinoma, and adenocarcinoma, but several other types occur less frequently. A few of the less common types are pleomorphic, carcinoid tumor, salivary gland carcinoma, and unclassified carcinoma. All types can occur in unusual histologic variants and as mixed cell-type combinations. Nonsquamous-cell carcinoma almost occupies the half of NSCLC. In the tissue classification, the cent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carcinoma In Situ
Carcinoma ''in situ'' (CIS) is a group of abnormal cells. While they are a form of neoplasm, there is disagreement over whether CIS should be classified as cancer. This controversy also depends on the exact CIS in question (i.e. cervical, skin, breast). Some authors do not classify them as cancer, however, recognizing that they can potentially become cancer. Others classify certain types as a non-invasive form of cancer. The term "pre-cancer" has also been used. These abnormal cells grow in their normal place, thus "''in situ''" (from Latin for "in its place"). For example, carcinoma ''in situ'' of the skin, also called Bowen's disease, is the accumulation of dysplastic epidermal cells within the epidermis only, that has failed to penetrate into the deeper dermis. For this reason, CIS will usually not form a tumor. Rather, the lesion is flat (in the skin, cervix, etc.) or follows the existing architecture of the organ (in the breast, lung, etc.). Exceptions include CIS of the colon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PDGFRA
PDGFRA, i.e. platelet-derived growth factor receptor A, also termed PDGFRα, i.e. platelet-derived growth factor receptor α, or CD140a i.e. Cluster of Differentiation 140a, is a receptor located on the surface of a wide range of cell types. This receptor binds to certain isoforms of platelet-derived growth factors (PDGFs) and thereby becomes active in stimulating cell signaling pathways that elicit responses such as cellular growth and differentiation. The receptor is critical for the development of certain tissues and organs during embryogenesis and for the maintenance of these tissues and organs, particularly hematologic tissues, throughout life. Mutations in the gene which codes for PDGFRA, i.e. the ''PDGFRA'' gene, are associated with an array of clinically significant neoplasms, notably ones of the clonal hypereosinophilia class of malignancies, as well as gastrointestinal stromal tumors (GISTs). Overall structure This gene encodes a typical receptor tyrosine kinase, which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NFE2L2
Nuclear factor erythroid 2-related factor 2 (NRF2), also known as nuclear factor erythroid-derived 2-like 2, is a transcription factor that in humans is encoded by the ''NFE2L2'' gene. NRF2 is a basic leucine zipper (bZIP) protein that may regulate the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation, according to preliminary research. In vitro, NRF2 binds to antioxidant response elements (AREs) in the promoter regions of genes encoding cytoprotective proteins. NRF2 induces the expression of heme oxygenase 1 ''in vitro'' leading to an increase in phase II enzymes. NRF2 also inhibits the NLRP3 inflammasome. NRF2 appears to participate in a complex regulatory network and performs a pleiotropic role in the regulation of metabolism, inflammation, autophagy, proteostasis, mitochondrial physiology, and immune responses. Several drugs that stimulate the NFE2L2 pathway are being studied for treatment of diseases that are ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
P110α
The phosphatidylinositol-4,5-bisphosphate 3-kinase, catalytic subunit alpha (the HUGO-approved official symbol = PIK3CA; HGNC ID, HGNC:8975), also called p110α protein, is a class I PI 3-kinase catalytic subunit. The human p110α protein is encoded by the ''PIK3CA'' gene. Its role was uncovered by molecular pathological epidemiology (MPE). Function Phosphatidylinositol-4,5-bisphosphate 3-kinase (also called phosphatidylinositol 3-kinase (PI3K)) is composed of an 85 kDa regulatory subunit and a 110 kDa catalytic subunit. The protein encoded by this gene represents the catalytic subunit, which uses ATP to phosphorylate phosphatidylinositols (PtdIns), PtdIns4P and PtdIns(4,5)P2. The involvement of p110α in human cancer has been hypothesized since 1995. Support for this hypothesis came from genetic and functional studies, including the discovery of common activating PIK3CA missense mutations in common human tumors. It has been found to be oncogenic and is implicated in cervi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
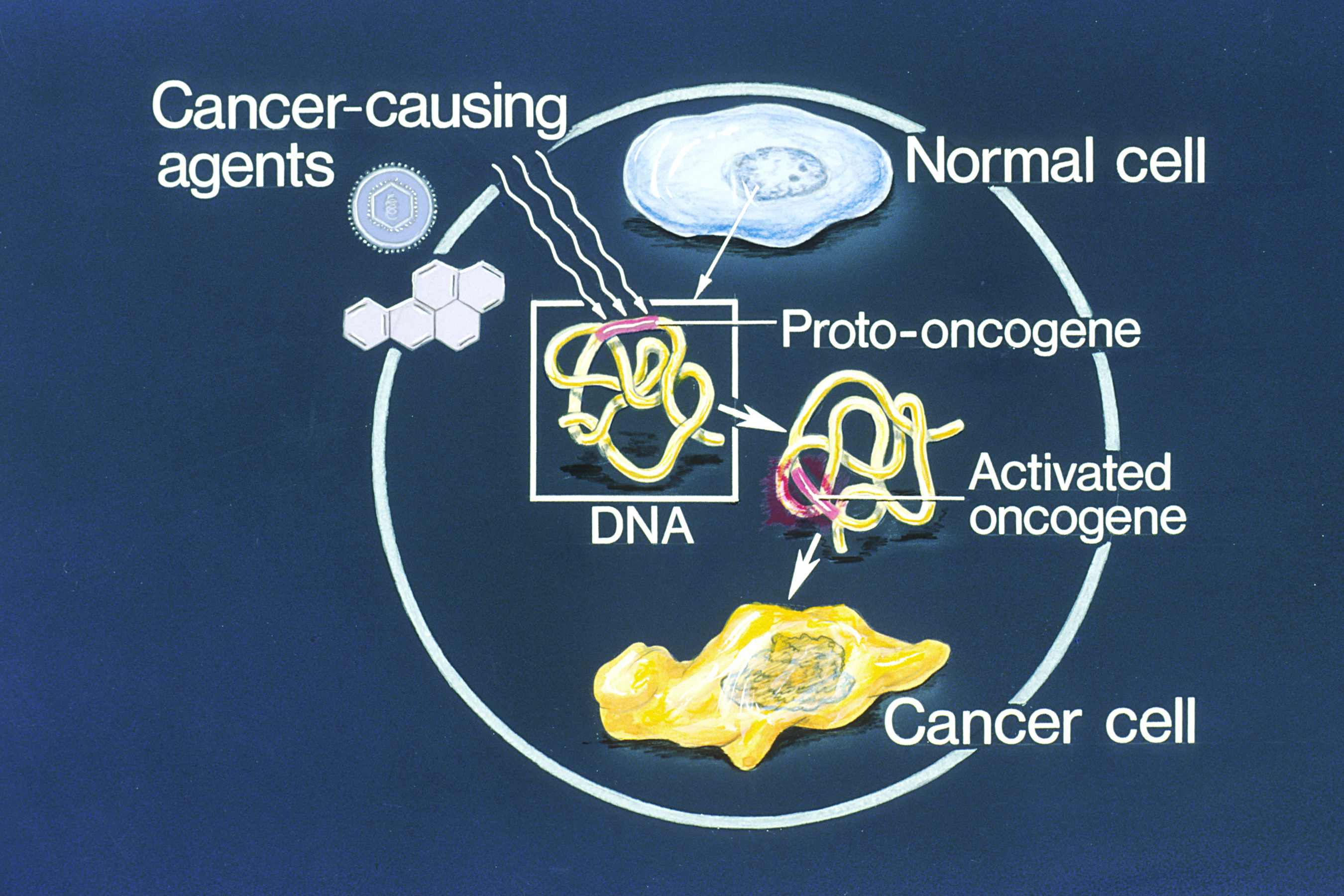
Oncogene
An oncogene is a gene that has the potential to cause cancer. In tumor cells, these genes are often mutated, or expressed at high levels.Kimball's Biology Pages. "Oncogenes" Free full text Most normal cells will undergo a programmed form of rapid cell death () when critical functions are altered and malfunctioning. Activated oncogenes can cause those cells designated for apoptosis to survive and proliferate instead. Most oncogenes began as proto-oncogenes: normal genes involved in cell growth and proliferation or inhibition of apoptosis. If, through mutation, normal genes promoting cellular growth are up-regulated (gain-of-function mutation), they will predisp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Notch 1
Neurogenic locus notch homolog protein 1 (Notch 1) is a protein encoded in humans by the ''NOTCH1'' gene. Notch 1 is a single-pass transmembrane receptor. Function This gene encodes a member of the Notch family. Members of this Type 1 transmembrane protein family share structural characteristics including an extracellular domain consisting of multiple epidermal growth factor-like (EGF) repeats, and an intracellular domain consisting of multiple, different domain types. Notch family members play a role in a variety of developmental processes by controlling cell fate decisions. The Notch signaling network is an evolutionarily conserved intercellular signaling pathway that regulates interactions between physically adjacent cells. In ''Drosophila'', notch interaction with its cell-bound ligands (delta, serrate) establishes an intercellular signaling pathway that plays a key role in development. Homologues of the notch-ligands have also been identified in humans, but precise interac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PTEN (gene)
Phosphatase and tensin homolog (PTEN) is a phosphatase in humans and is encoded by the ''PTEN'' gene. Mutations of this gene are a step in the development of many cancers, specifically glioblastoma, lung cancer, breast cancer, and prostate cancer. Genes corresponding to PTEN (orthologs) have been identified in most mammals for which complete genome data are available. ''PTEN'' acts as a tumor suppressor gene through the action of its phosphatase protein product. This phosphatase is involved in the regulation of the cell cycle, preventing cells from growing and dividing too rapidly. It is a target of many anticancer drugs. The protein encoded by this gene is a phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase. It contains a tensin-like domain as well as a catalytic domain similar to that of the dual specificity protein tyrosine phosphatases. Unlike most of the protein tyrosine phosphatases, this protein preferentially dephosphorylates phosphoinositide substrates. It nega ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
KEAP1
Kelch-like ECH-associated protein 1 is a protein that in humans is encoded by the ''Keap1'' gene. Structure Keap1 has four discrete protein domains. The N-terminal Broad complex, Tramtrack and Bric-à-Brac (BTB) domain contains the Cys151 residue, which is one of the important cysteines in stress sensing. The intervening region (IVR) domain contains two critical cysteine residues, Cys273 and Cys288, which are a second group of cysteines important for stress sensing. A double glycine repeat (DGR) and C-terminal region (CTR) domains collaborate to form a β-propeller structure, which is where Keap1 interacts with Nrf2. Interactions Keap1 has been shown to interact with Nrf2, a master regulator of the antioxidant response, which is important for the amelioration of oxidative stress. Under quiescent conditions, Nrf2 is anchored in the cytoplasm through binding to Keap1, which, in turn, facilitates the ubiquitination and subsequent proteolysis of Nrf2. Such sequestratio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CDKN2A
CDKN2A, also known as cyclin-dependent kinase inhibitor 2A, is a gene which in humans is located at chromosome 9, band p21.3. It is ubiquitously expressed in many tissues and cell types. The gene codes for two proteins, including the INK4 family member p16 (or p16INK4a) and p14arf. Both act as tumor suppressors by regulating the cell cycle. p16 inhibits cyclin dependent kinases 4 and 6 ( CDK4 and CDK6) and thereby activates the retinoblastoma (Rb) family of proteins, which block traversal from G1 to S-phase. p14ARF (known as p19ARF in the mouse) activates the p53 tumor suppressor. Somatic mutations of CDKN2A are common in the majority of human cancers, with estimates that CDKN2A is the second most commonly inactivated gene in cancer after p53. Germline mutations of CDKN2A are associated with familial melanoma, glioblastoma and pancreatic cancer. The ''CDKN2A'' gene also contains one of 27 SNPs associated with increased risk of coronary artery disease. Structure Gene The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
KMT2D
Histone-lysine N-methyltransferase 2D (KMT2D), also known as MLL4 and sometimes MLL2 in humans and Mll4 in mice, is a major mammalian histone H3 lysine 4 (H3K4) mono-methyltransferase. It is part of a family of six Set1-like H3K4 methyltransferases that also contains KMT2A (or MLL1), KMT2B (or MLL2), KMT2C (or MLL3), KMT2F (or SET1A), and KMT2G (or SET1B). KMT2D is a large protein over 5,500 amino acids in size and is widely expressed in adult tissues. The protein co-localizes with lineage determining transcription factors on transcriptional enhancers and is essential for cell differentiation and embryonic development. It also plays critical roles in regulating cell fate transition, metabolism, and tumor suppression. Mutations in KMT2D cause human genetic conditions including Kabuki syndrome, another distinct congenital malformations disorder and various forms of cancer. Structure Gene In mice, KMT2D is coded by the ''Kmt2d'' gene located on chromosome 15F1. Its transc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |