|
NFE2L2
Nuclear factor erythroid 2-related factor 2 (NRF2), also known as nuclear factor erythroid-derived 2-like 2, is a transcription factor that in humans is encoded by the ''NFE2L2'' gene. NRF2 is a basic leucine zipper (bZIP) protein that may regulate the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation, according to preliminary research. In vitro, NRF2 binds to antioxidant response elements (AREs) in the promoter regions of genes encoding cytoprotective proteins. NRF2 induces the expression of heme oxygenase 1 ''in vitro'' leading to an increase in phase II enzymes. NRF2 also inhibits the NLRP3 inflammasome. NRF2 appears to participate in a complex regulatory network and performs a pleiotropic role in the regulation of metabolism, inflammation, autophagy, proteostasis, mitochondrial physiology, and immune responses. Several drugs that stimulate the NFE2L2 pathway are being studied for treatment of diseases that are ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
NFE2L3
Nuclear factor (erythroid 2)-like factor 3, also known as NFE2L3 or 'NRF3', is a transcription factor that in humans is encoded by the ''Nfe2l3'' gene. Nrf3 is a basic leucine zipper (bZIP) transcription factor belonging to the Cap ‘n’ Collar (CNC) family of proteins. In 1989, the first CNC transcription factor NFE2L2 was identified. Subsequently, several related proteins were identified, including NFE2L1 and NFE2L3, in different organisms such as humans, mice, and zebrafish. These proteins are specifically encoded in the humans by ''Nfe2l1'' and ''Nfe2l3'' genes respectively. Gene The ''Nfe2l3'' gene was mapped to the chromosomal location 7p15-p14 by fluorescence in situ hybridization (FISH). It covers 34.93 kB from base 26191830 to 26226754 on the direct DNA strand with an exon count of 4. The gene is found near the HOXA gene cluster, similar to the clustering of p45 NF-E2, NFE2L1, and NFE2L2 near HOXC, HOXB, and HOXD genes respectively. This implies that all four genes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BZIP Domain
The Basic Leucine Zipper Domain (bZIP domain) is found in many DNA binding eukaryotic proteins. One part of the domain contains a region that mediates sequence specific DNA binding properties and the leucine zipper that is required to hold together (dimerize) two DNA binding regions. The DNA binding region comprises a number of basic amino acids such as arginine and lysine. Proteins containing this domain are transcription factors. bZIP transcription factors bZIP transcription factors are found in all eukaryotes and form one of the largest families of dimerizing TFs. An evolutionary study from 2008 revealed that 4 bZIP genes were encoded by the genome of the most recent common ancestor of all plants. Interactions between bZIP transcription factors are numerous and complex and play important roles in cancer development in epithelial tissues, steroid hormone synthesis by cells of endocrine tissues, factors affecting reproductive functions, and several other phenomena that aff ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
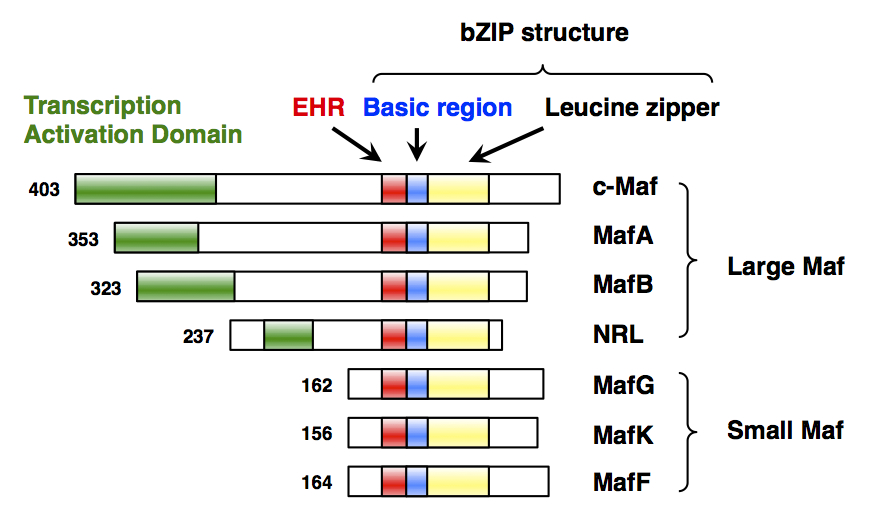
MAFG
Transcription factor MafG is a bZip Maf transcription factor protein that in humans is encoded by the ''MAFG'' gene. MafG is one of the small Maf proteins, which are basic region and leucine zipper (bZIP)-type transcription factors. The HUGO Gene Nomenclature Committee-approved gene name of ''MAFG'' is “v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog G”. Discovery MafG was first cloned and identified in chicken in 1995 as a new member of the small Maf (sMaf) genes. MAFG has been identified in many vertebrates, including humans. There are three functionally redundant sMaf proteins in vertebrates, MafF, MafG, and MafK. Structure MafG has a bZIP structure that consists of a basic region for DNA binding and a leucine zipper structure for dimer formation. Similar to other sMafs, MafG lacks any canonical transcriptional activation domains. Expression ''MAFG'' is broadly but differentially expressed in various tissues. ''MAFG'' expression was detected in all ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MAFF (gene)
Transcription factor MafF is a bZip Maf transcription factor protein that in humans is encoded by the ''MAFF'' gene. MafF is one of the small Maf proteins, which are basic region and leucine zipper (bZIP)-type transcription factors. The HUGO Gene Nomenclature Committee-approved gene name of ''MAFF'' is “v-maf avian musculoaponeurotic fibrosarcoma oncogene homolog F”. Discovery MafF was first cloned and identified in chicken in 1993 as a member of the small Maf (sMaf) genes. MAFF has been identified in many vertebrates, including humans. There are three functionally redundant sMaf proteins in vertebrates, MafF, MafG, and MafK. Structure MafF has a bZIP structure that consists of a basic region for DNA binding and a leucine zipper structure for dimer formation. Similar to other sMafs, MafF lacks any canonical transcriptional activation domains. Expression ''MAFF'' is broadly but differentially expressed in various tissues. ''MAFF'' expression was detected in all 16 t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
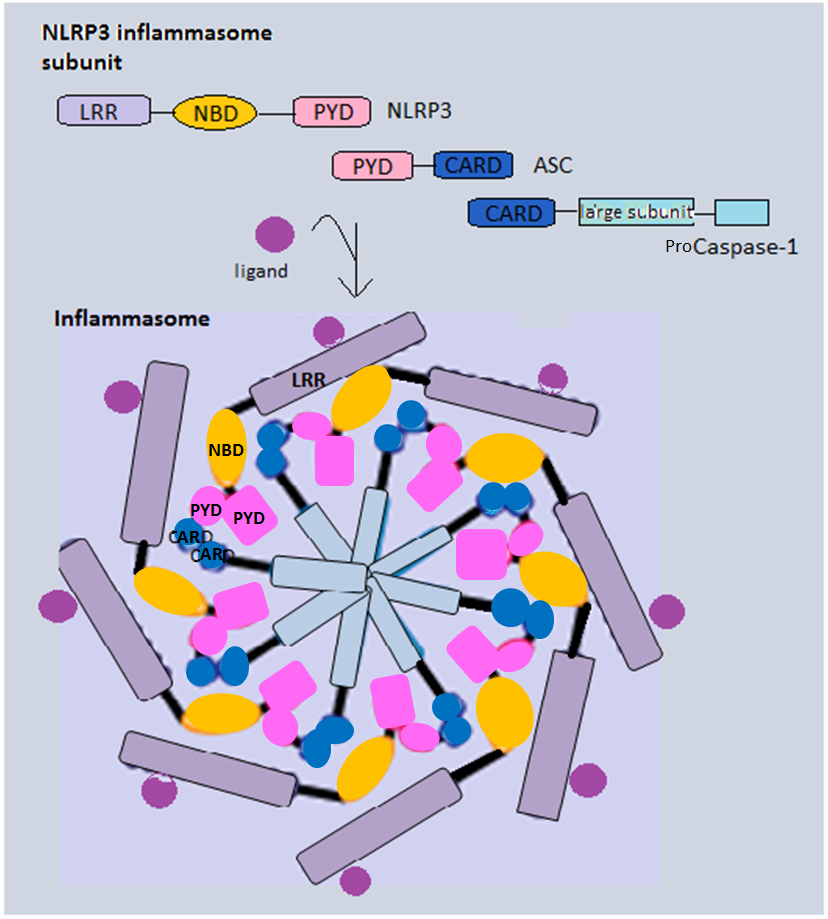
Inflammasome
Inflammasomes are cytosolic multiprotein oligomers of the innate immune system responsible for the activation of inflammatory responses. Activation and assembly of the inflammasome promotes proteolytic cleavage, maturation and secretion of pro-inflammatory cytokines interleukin 1β (IL-1β) and interleukin 18 (IL-18), as well as cleavage of Gasdermin-D. The N-terminal fragment resulting from this cleavage induces a pro-inflammatory form of programmed cell death distinct from apoptosis, referred to as pyroptosis, and is responsible for secretion of the mature cytokines, presumably through the formation of pores in the plasma membrane. Inflammasome activation is initiated by different kinds of cytosolic pattern recognition receptors (PRRs) that respond to either microbe-derived pathogen-associated molecular patterns (PAMPs) or danger-associated molecular patterns (DAMPs) generated by the host cell. Pattern recognition receptors involved in inflammasomes comprise NLRs (nucleoti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-jun
Transcription factor Jun is a protein that in humans is encoded by the ''JUN'' gene. c-Jun, in combination with protein c-Fos, forms the AP-1 early response transcription factor. It was first identified as the Fos-binding protein p39 and only later rediscovered as the product of the JUN gene. c-jun was the first oncogenic transcription factor discovered. The proto-oncogene c-Jun is the cellular homolog of the viral oncoprotein v-jun (). The viral homolog v-jun was discovered in avian sarcoma virus 17 and was named for ''ju-nana'', the Japanese word for 17. The human JUN encodes a protein that is highly similar to the viral protein, which interacts directly with specific target DNA sequences to regulate gene expression. This gene is intronless and is mapped to 1p32-p31, a chromosomal region involved in both translocations and deletions in human malignancies. Function Regulation Both Jun and its dimerization partners in AP-1 formation are subject to regulation by diverse extr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cullin 3
Cullin 3 is a protein that in humans is encoded by the ''CUL3'' gene. Cullin 3 protein belongs to the family of cullins which in mammals contains eight proteins (Cullin 1, Cullin 2, Cullin 3, Cullin 4A, Cullin 4B, Cullin 5, Cullin 7 and Cullin 9). Cullin proteins are an evolutionarily conserved family of proteins throughout yeast, plants and mammals. Protein function Cullin 3 is a component of Cullin-RING E3 ubiquitin ligases complexes (CRLs) which are involved in protein ubiquitylation and represent a part of ubiquitin–proteasome system (UPS). Added ubiquitin moieties to the lysine residue by CRLs then target the protein for the proteasomal degradation. Cullin-RING E3 ubiquitin ligases are involved in many cellular processes responsible for cell cycle regulation, stress response, protein trafficking, signal transduction, DNA replication, transcription, protein quality control, circadian clock and development. Deletion of ''CUL3'' gene in mice causes embryonic lethality. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
KEAP1
Kelch-like ECH-associated protein 1 is a protein that in humans is encoded by the ''Keap1'' gene. Structure Keap1 has four discrete protein domains. The N-terminal Broad complex, Tramtrack and Bric-à-Brac (BTB) domain contains the Cys151 residue, which is one of the important cysteines in stress sensing. The intervening region (IVR) domain contains two critical cysteine residues, Cys273 and Cys288, which are a second group of cysteines important for stress sensing. A double glycine repeat (DGR) and C-terminal region (CTR) domains collaborate to form a β-propeller structure, which is where Keap1 interacts with Nrf2. Interactions Keap1 has been shown to interact with Nrf2, a master regulator of the antioxidant response, which is important for the amelioration of oxidative stress. Under quiescent conditions, Nrf2 is anchored in the cytoplasm through binding to Keap1, which, in turn, facilitates the ubiquitination and subsequent proteolysis of Nrf2. Such sequestratio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-Fos
Protein c-Fos is a proto-oncogene that is the human homolog of the retroviral oncogene v-fos. It is encoded in humans by the ''FOS'' gene. It was first discovered in rat fibroblasts as the transforming gene of the FBJ MSV (Finkel–Biskis–Jinkins murine osteogenic sarcoma virus) (Curran and Tech, 1982). It is a part of a bigger Fos family of transcription factors which includes c-Fos, FosB, Fra-1 and Fra-2. It has been mapped to chromosome region 14q21→q31. c-Fos encodes a 62 kDa protein, which forms heterodimer with c-jun (part of Jun family of transcription factors), resulting in the formation of AP-1 (Activator Protein-1) complex which binds DNA at AP-1 specific sites at the promoter and enhancer regions of target genes and converts extracellular signals into changes of gene expression. It plays an important role in many cellular functions and has been found to be overexpressed in a variety of cancers. Structure and function c-Fos is a 380 amino acid protein with a basic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CREB
CREB-TF (CREB, cAMP response element-binding protein) is a cellular transcription factor. It binds to certain DNA sequences called cAMP response elements (CRE), thereby increasing or decreasing the transcription of the genes. CREB was first described in 1987 as a cAMP-responsive transcription factor regulating the somatostatin gene. Genes whose transcription is regulated by CREB include: '' c-fos'', BDNF, tyrosine hydroxylase, numerous neuropeptides (such as somatostatin, enkephalin, VGF, corticotropin-releasing hormone), and genes involved in the mammalian circadian clock (PER1, PER2). CREB is closely related in structure and function to CREM (cAMP response element modulator) and ATF-1 (activating transcription factor-1) proteins. CREB proteins are expressed in many animals, including humans. CREB has a well-documented role in neuronal plasticity and long-term memory formation in the brain and has been shown to be integral in the formation of spatial memory. CREB downreg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucine Zipper
A leucine zipper (or leucine scissors) is a common three-dimensional structural motif in proteins. They were first described by Landschulz and collaborators in 1988 when they found that an enhancer binding protein had a very characteristic 30-amino acid segment and the display of these amino acid sequences on an idealized alpha helix revealed a periodic repetition of leucine residues at every seventh position over a distance covering eight helical turns. The polypeptide segments containing these periodic arrays of leucine residues were proposed to exist in an alpha-helical conformation and the leucine side chains from one alpha helix interdigitate with those from the alpha helix of a second polypeptide, facilitating dimerization. Leucine zippers are a dimerization motif of the bZIP (Basic-region leucine zipper) class of eukaryotic transcription factors. The bZIP domain is 60 to 80 amino acids in length with a highly conserved DNA binding basic region and a more diversified leucin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ubiquitination
Ubiquitin is a small (8.6 kDa) regulatory protein found in most tissues of eukaryotic organisms, i.e., it is found ''ubiquitously''. It was discovered in 1975 by Gideon Goldstein and further characterized throughout the late 1970s and 1980s. Four genes in the human genome code for ubiquitin: UBB, UBC, UBA52 and RPS27A. The addition of ubiquitin to a substrate protein is called ubiquitylation (or, alternatively, ubiquitination or ubiquitinylation). Ubiquitylation affects proteins in many ways: it can mark them for degradation via the proteasome, alter their cellular location, affect their activity, and promote or prevent protein interactions. Ubiquitylation involves three main steps: activation, conjugation, and ligation, performed by ubiquitin-activating enzymes (E1s), ubiquitin-conjugating enzymes (E2s), and ubiquitin ligases (E3s), respectively. The result of this sequential cascade is to bind ubiquitin to lysine residues on the protein substrate via an isopeptide bond, cy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |