|
Sodium-transporting Carboxylic Acid Decarboxylase
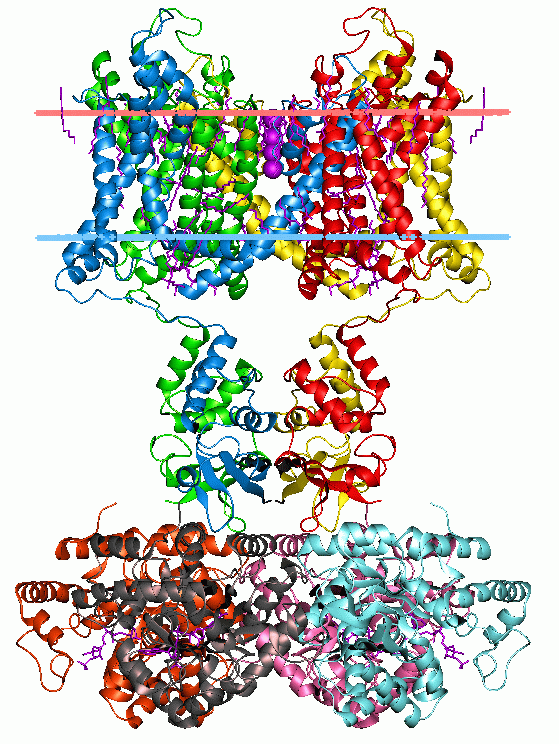
The Na+-transporting Carboxylic Acid Decarboxylase (NaT-DC) FamilyTC# 3.B.1 is a family of porters that belong to the CPA superfamily. Members of this family have been characterized in both Gram-positive and Gram-negative bacteria. A representative list of proteins belonging to the NaT-DC family can be found in thTransporter Classification Database Function Porters of the NaT-DC family catalyze decarboxylation of a substrate carboxylic acid and use the energy released to drive extrusion of one or two sodium ions (Na+) from the cytoplasm of the cell. These systems have been characterized only from bacteria. The generalized reaction for the NaT-DC family is:R - CO (in) + H+ (out) and 1 or 2 Na+ (in) ←→ R-H + CO2 (in) and 1 or 2 Na+ (out).Distinct enzymes catalyze decarboxylation of (1) oxaloacetate, (2) methylmalonyl-CoA, (3) glutaconyl-CoA and (4) malonate. The oxaloacetate decarboxylases (EC 4.1.1.3TC# 3.B.1.1.1, methylmalonyl CoA decarboxylases (EC 4.1.1.4TC# 3.B.1.1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CPA Superfamily
The cation:proton antiporter (CPA) superfamily is a superfamily of transport proteins named after one of its constituent members, the monovalent cation:proton antiporter-2 (CPA2). CPA1 was considered a member of the superfamily until 2010, when it was discovered to in fact be a member of the VIC Vic (; es, Vic or Pancracio Celdrán (2004). Diccionario de topónimos españoles y sus gentilicios (5ª edición). Madrid: Espasa Calpe. p. 843. ISBN 978-84-670-3054-9. «Vic o Vich (viquense, vigitano, vigatán, ausense, ausetano, ausonense): ... superfamily. As of April 2016, the CPA superfamily consists of four members: * Monovalent cation:proton antiporter-2 family * Malonate:Sodium symporter family * Putative sulfate exporter family * Sodium-transporting carboxylic acid decarboxylase family Structural variation exists between families. For example, members of the CPA2 family are between 300 and 900 amino acyl residues (aas) in length and exhibit 10 to 14 transmembrane segme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrococcus Abyssi
''Pyrococcus abyssi'' is a hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent in the North Fiji Basin at . It is anaerobic, sulfur-metabolizing, gram-negative, coccus-shaped and highly motile. Its optimum growth temperature is . Its type strain is GE5 (CNCM I-1302). ''Pyrococcus abyssi'' has been used as a model organism in studies of DNA polymerase. This species can also grow at high cell densities in bioreactors. References Further reading * *Cohen, Georges N., et al. "An integrated analysis of the genome of the hyperthermophilic archaeon Pyrococcus abyssi." Molecular microbiology 47.6 (2003): 1495–1512. * External links *Type strain of ''Pyrococcus abyssi'' at Bac''Dive'' - the Bacterial Diversity Metadatabase Archaea described in 1993 Euryarchaeota {{Euryarchaeota-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Proteins
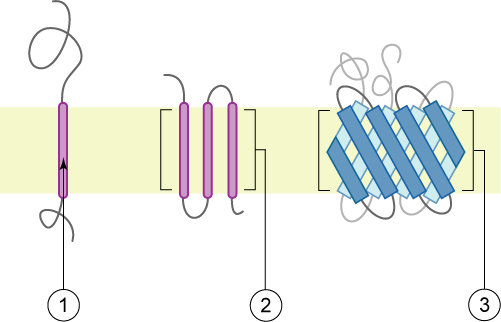
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (transmembrane) or associate with one or the other side of a membrane ( integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane. Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs. Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct conformation of the protein in isolation from its native environment. Function Membrane proteins perform a variety of functions vital to the surv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Families
A protein family is a group of evolutionarily related proteins. In many cases, a protein family has a corresponding gene family, in which each gene encodes a corresponding protein with a 1:1 relationship. The term "protein family" should not be confused with family as it is used in taxonomy. Proteins in a family descend from a common ancestor and typically have similar three-dimensional structures, functions, and significant sequence similarity. The most important of these is sequence similarity (usually amino-acid sequence), since it is the strictest indicator of homology and therefore the clearest indicator of common ancestry. A fairly well developed framework exists for evaluating the significance of similarity between a group of sequences using sequence alignment methods. Proteins that do not share a common ancestor are very unlikely to show statistically significant sequence similarity, making sequence alignment a powerful tool for identifying the members of protein familie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Protein
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (transmembrane) or associate with one or the other side of a membrane ( integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane. Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs. Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct conformation of the protein in isolation from its native environment. Function Membrane proteins perform a variety of functions vital to the sur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decarboxylase
Carboxy-lyases, also known as decarboxylases, are carbon–carbon lyases that add or remove a carboxyl group from organic compounds. These enzymes catalyze the decarboxylation of amino acids, beta-keto acids and alpha-keto acids. Classification and nomenclature Carboxy-lyases are categorized under EC number 4.1.1. Usually, they are named after the substrate whose decarboxylation they catalyze, for example pyruvate decarboxylase catalyzes the decarboxylation of pyruvate. Examples * Aromatic-L-amino-acid decarboxylase * Glutamate decarboxylase * Histidine decarboxylase * Ornithine decarboxylase * Phosphoenolpyruvate carboxylase * Pyruvate decarboxylase * RuBisCO – the only carboxylase that leads to a net fixation of carbon dioxide * Uridine monophosphate synthetase * Uroporphyrinogen III decarboxylase * enoyl-CoA carboxylases/reductases (ECRs) See also * Enzymes * Lyase In biochemistry, a lyase is an enzyme that catalyzes the breaking (an elimination reaction) of various chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxybiotin Decarboxylase
Carboxybiotin decarboxylase (, ''MadB'', ''carboxybiotin protein decarboxylase'') is an enzyme with systematic name ''carboxybiotinyl-(protein) carboxy-lyase''. This enzyme catalyses the following chemical reaction : a carboxybiotinyl- rotein+ ''n'' Na+in + H+out \rightleftharpoons CO2 + a biotinyl- rotein+ ''n'' Na+out (''n'' = 1--2) This enzyme is an integral membrane protein MadB from the anaerobic bacterium '' Malonomonas rubra''. Nomenclature This enzyme was previously classified as . References External links * {{Portal bar, Biology, border=no EC 4.3.99 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biocytin
Biocytin is a chemical compound that is an amide formed from the vitamin biotin and the amino acid L-lysine. As an intermediate in the metabolism of biotin, biocytin occurs naturally in blood serum and urine. The enzyme biotinidase cleaves biocytin and makes biotin available to be reused by other enzymes. Because biocytin is the natural substrate of the enzyme biotinidase, biocytin can be used to measure the biotinidase activity and therefore diagnose biotinidase deficiency. Biocytin is also used in scientific research as a histological stain Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology (microscopic study of biological tissues), in cytology (microscopic study of cells), and in the ... for nerve cells. References {{reflist Carboxamides Amino acid derivatives Histology ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TIM Barrel
The TIM barrel (triose-phosphate isomerase), also known as an alpha/beta barrel, is a conserved protein fold consisting of eight alpha helices (α-helices) and eight parallel beta strands (β-strands) that alternate along the peptide backbone. The structure is named after triose-phosphate isomerase, a conserved metabolic enzyme. TIM barrels are ubiquitous, with approximately 10% of all enzymes adopting this fold. Further, five of seven enzyme commission (EC) enzyme classes include TIM barrel proteins. The TIM barrel fold is evolutionarily ancient, with many of its members possessing little similarity today, instead falling within the ''twilight zone'' of sequence similarity. The inner beta barrel (β-barrel) is in many cases stabilized by intricate salt-bridge networks. Loops at the C-terminal ends of the β-barrel are responsible for catalytic activity while N-terminal end loops are important for the stability of the TIM-barrels. Structural inserts ranging from extended loo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxyl Transferase Domain
In molecular biology, proteins containing the carboxyl transferase domain include biotin-dependent carboxylases. This domain carries out the following reaction: transcarboxylation from biotin to an acceptor molecule. There are two recognised types of carboxyl transferase. One of them uses acyl-CoA Acyl-CoA is a group of coenzymes that metabolize fatty acids. Acyl-CoA's are susceptible to beta oxidation, forming, ultimately, acetyl-CoA. The acetyl-CoA enters the citric acid cycle, eventually forming several equivalents of ATP. In this way ... and the other uses 2-oxo acid as the acceptor molecule of carbon dioxide. All of the members in this family use acyl-CoA as the acceptor molecule. References {{InterPro content, IPR000022 Protein families ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propionyl-CoA Carboxylase
Propionyl-CoA carboxylase (, PCC) catalyses the carboxylation reaction of propionyl-CoA in the mitochondrial matrix. PCC has been classified both as a ligase and a lyase. The enzyme is biotin-dependent. The product of the reaction is (S)-methylmalonyl CoA. : ATP + propionyl-CoA + HCO3− ADP + phosphate + (S)-methylmalonyl-CoA (S)-Methylmalonyl-CoA cannot be directly utilized by animals. It is acted upon by a racemase, yielding (R)-methylmalonyl-CoA, which is then converted into succinyl-CoA by methylmalonyl-CoA mutase (one of the few metabolic enzymes which requires vitamin B12 as a cofactor). Succinyl-CoA, a Krebs cycle intermediate, is further metabolized into fumarate, then malate, and then oxaloacetate. Oxaloacetate may be transported into the cytosol to form phosphoenol pyruvate and other gluconeogenic intermediates. Propionyl-CoA is therefore an important precursor to glucose. Propionyl-CoA is the end product of odd-chain fatty acid metabolism, including most methylated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |