|
Silyl Hydride
Hydrosilanes are tetravalent silicon compounds containing one or more Si-H bond. The parent hydrosilane is silane (SiH4). Commonly, hydrosilane refers to organosilicon derivatives. Examples include phenylsilane (PhSiH3) and triethoxysilane ((C2H5O)3SiH). Polymers and oligomers terminated with hydrosilanes are resins that are used to make useful materials like caulks. Synthesis Trichlorosilane is produced commercially by the reaction of hydrogen chloride with silicon: :Si + 3 HCl → HSiCl3 + H2 Many alkoxy hydrosilanes are generated by alcoholysis of trichlorosilane. One example is triethoxysilane: :HSiCl3 + 3EtOH → HSi(OEt)3 + 3 HCl Organohydrosilanes can be prepared by partial hydrosilation of silane itself: :SiH4 + 3 C2H4 → HSi(C2H5)3 In the laboratory, hydrosilanes classically are prepared by treating chlorosilanes with hydride reagents, such as lithium aluminium hydride: :4ClSi(C2H5)3 + LiAlH4 → 4HSi(C2H5)3 + LiAlCl4 Structure The silicon-to-hydrogen bond is longer t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silane
Silane is an inorganic compound with chemical formula, . It is a colourless, pyrophoric, toxic gas with a sharp, repulsive smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silane with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents. Production Commercial-scale routes Silane can be produced by several routes. Typically, it arises from the reaction of hydrogen chloride with magnesium silicide: : Mg2Si + 4 HCl -> 2 MgCl2 + SiH4 It is also prepared from metallurgical-grade silicon in a two-step process. First, silicon is treated with hydrogen chloride at about 300 °C to produce trichlorosilane, HSiCl3, along with hydrogen gas, according to the chemical equation : Si + 3 HCl -> HSiCl3 + H2 The trichlorosilane is then converted to a mixture of silane and silicon tetrachloride: ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrogen Complex
Dihydrogen complexes are coordination complexes containing intact H2 as a ligand. They are a subset of sigma complexes. The prototypical complex is W(CO)3( PCy3)2(H2). This class of compounds represent intermediates in metal-catalyzed reactions involving hydrogen. Hundreds of dihydrogen complexes have been reported. Most examples are cationic transition metals complexes with octahedral geometry. Upon complexation, the H−H bond is extended to 0.81–0.82 Å as indicated by neutron diffraction, about a 10% extension relative to the H−H bond in free H2. Some complexes containing multiple hydrogen ligands, i.e. polyhydrides, also exhibit short H−H contacts. It has been suggested that distances 1 Å are better described as dihydride complexes (see figure). Characterization The usual method for characterization is 1H NMR spectroscopy. The magnitude of spin-spin coupling, ''J''HD, is a useful indicator of the strength of the bond between the hydrogen and deuterium i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
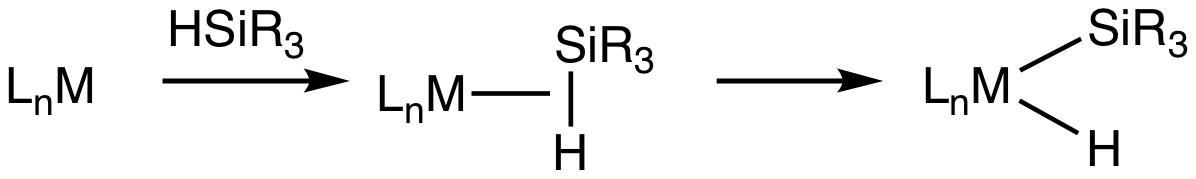
Transition Metal Silane Complexes
Transition metal silane complexes are coordination compounds containing hydrosilane ligands. An early example is (MeC5H4)Mn(CO)2(η2-HSiPh3) (Ph = C6H5). The bonding in silane sigma complexes is similar to that invoked in agostic interactions. The metal center engages the Si-H entity via a 3-center, 2-electron bond. It is widely assumed that these sigma complexes are intermediates in the oxidative addition of hydrosilanes to give metal silyl hydrides. This transformation is invoked in hydrosilylation catalysis. Evidence for sigma-silane complexes is provided by proton NMR spectroscopy. For (MeC5H4)Mn(CO)2(η2-HSiPh3), J(29Si,1H) = 65 Hz compared to 180 Hz in free diphenylsilane. In silyl hydride complexes, the coupling in about 6 Hz. Neutron diffraction Neutron diffraction or elastic neutron scattering is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material. A sample to be examined is placed in a beam of therma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionic Hydrogenations
Ionic hydrogenation refers to hydrogenation achieved by the addition of a hydride to substrate that has been activated by an electrophile. Some ionic hydrogenations entail addition of H2 to the substrate and some entail replacement of a heteroatom with hydride. Traditionally, the method was developed for acid-induced reductions with hydrosilanes. Alternatively ionic hydrogenation can be achieved using H2. Bullock, R. M. "Ionic Hydrogenations," in The Handbook of Homogeneous Hydrogenation (eds J. G. de Vries and C. J. Elsevier), Wiley-VCH Verlag GmbH, Weinheim, Germany, 2007. Ionic hydrogenation is employed when the substrate can produce a stable carbonium ion. Polar double bonds are favored substrates. Using hydrosilanes Because silicon (1.90) is more electropositive than hydrogen (2.20), hydrosilanes exhibit (mild) hydridic character. Hydrosilanes can serve as hydride donors to highly electrophilic organic substrates. Many alcohols, alkyl halides, acetals, orthoesters, alkenes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Deoxygenation
Deoxygenation is a chemical reaction involving the removal of oxygen atoms from a molecule. The term also refers to the removal of molecular oxygen (O2) from gases and solvents, a step in air-free technique and gas purifiers. As applied to organic compounds, deoxygenation is a component of fuels production as well a type of reaction employed in organic synthesis, e.g. of pharmaceuticals. Deoxygenation of C-O bonds With replacement by H2 The main examples involving the replacement of an oxo group by two hydrogen atoms (A=O → AH2) are hydrogenolysis. Typical examples use metal catalysts and H2 as the reagent. Conditions are typically more forcing than hydrogenation. Stoichiometric reactions that effect deoxygenation include the Wolff–Kishner reduction for aryl ketones. The replacement of a hydroxyl group by hydrogen (A-OH → A-H) is the point of the Barton–McCombie deoxygenation and the Markó–Lam deoxygenation. Biomass valorization Deoxygenation is an impo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reductions With Hydrosilanes
Reductions with hydrosilanes are methods used for hydrogenations and hydrogenolysis of organic compounds. The approach is a subset of Ionic hydrogenations. In this particular method, the substrate is treated with a hydrosilane and auxiliary reagent, often a strong acid, resulting in formal transfer of hydride from silicon to carbon. This style of reduction with hydrosilanes enjoys diverse if specialized applications. Scope Deoxygenation of alcohols and halides Some alcohols are reduced to alkanes when treated with hydrosilanes in the presence of a strong Lewis acid. Brønsted acids may also be used. Tertiary alcohols undergo facile reduction using boron trifluoride etherate as the Lewis acid. Primary alcohols require an excess of the silane, a stronger Lewis acid, and long reaction times. : Skeletal rearrangements are sometimes induced. Another side reaction is nucleophilic attack of the conjugate base on the intermediate carbocation. In organosilane reductions of substrates bear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borohydride
Borohydride refers to the anion , which is also called tetrahydroborate, and its salts. Borohydride or hydroborate is also the term used for compounds containing , where ''n'' is an integer from 0 to 3, for example cyanoborohydride or cyanotrihydroborate and triethylborohydride or triethylhydroborate . Borohydrides find wide use as reducing agents in organic synthesis. The most important borohydrides are lithium borohydride and sodium borohydride, but other salts are well known (see Table). Tetrahydroborates are also of academic and industrial interest in inorganic chemistry. History Alkali metal borohydrides were first described in 1940 by Hermann Irving Schlesinger and Herbert C. Brown. They synthesized lithium borohydride from diborane : :, where M = Li, Na, K, Rb, Cs, etc. Current methods involve reduction of trimethyl borate with sodium hydride. Structure In the borohydride anion and most of its modifications, boron has a tetrahedral structure. The reactivity of the B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |