Transition Metal Silane Complexes on:
[Wikipedia]
[Google]
[Amazon]
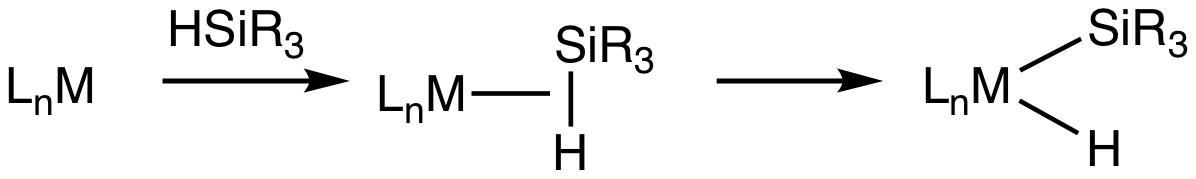 Transition metal silane complexes are
Transition metal silane complexes are  Evidence for sigma-silane complexes is provided by
Evidence for sigma-silane complexes is provided by
 Transition metal silane complexes are
Transition metal silane complexes are coordination compound
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
s containing hydrosilane ligands
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electro ...
. An early example is (MeC5H4)Mn(CO)2(η2-HSiPh3) (Ph = C6H5).
The bonding in silane sigma complexes is similar to that invoked in agostic interaction
In organometallic chemistry, agostic interaction refers to the interaction of a coordinatively-unsaturated transition metal with a C−H bond, when the two electrons involved in the C−H bond enter the empty d-orbital of the transition metal, r ...
s. The metal center engages the Si-H entity via a 3-center, 2-electron bond. It is widely assumed that these sigma complexes are intermediates in the oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidat ...
of hydrosilanes to give metal silyl hydrides. This transformation is invoked in hydrosilylation Hydrosilylation, also called catalytic hydrosilation, describes the addition of Si-H bonds across unsaturated bonds."Hydrosilylation A Comprehensive Review on Recent Advances" B. Marciniec (ed.), Advances in Silicon Science, Springer Science, 2009 ...
catalysis.
 Evidence for sigma-silane complexes is provided by
Evidence for sigma-silane complexes is provided by proton NMR spectroscopy
Proton nuclear magnetic resonance (proton NMR, hydrogen-1 NMR, or 1H NMR) is the application of nuclear magnetic resonance in NMR spectroscopy with respect to hydrogen-1 nuclei within the molecules of a substance, in order to determine the struct ...
. For (MeC5H4)Mn(CO)2(η2-HSiPh3), J(29Si,1H) = 65 Hz compared to 180 Hz in free diphenylsilane. In silyl hydride complexes, the coupling in about 6 Hz. Neutron diffraction
Neutron diffraction or elastic neutron scattering is the application of neutron scattering to the determination of the atomic and/or magnetic structure of a material. A sample to be examined is placed in a beam of thermal or cold neutrons to o ...
studies reveal a Si-H distance of 1.802(5) Å in the corresponding η2-HSiFPh2 complex vs 1.48 Å in free HSiFPh2. Elongated Si-H bonds are characteristic of these sigma complexes.
References