|
Silicaceous
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , most commonly found in nature as quartz and in various living organisms. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and most abundant families of materials, existing as a compound of several minerals and as a synthetic product. Notable examples include fused quartz, fumed silica, silica gel, opal and aerogels. It is used in structural materials, microelectronics (as an electrical insulator), and as components in the food and pharmaceutical industries. Structure In the majority of silicates, the silicon atom shows tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. In contrast, CO2 is a linear molecule. The starkly different structures of the dio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Birefringence
Birefringence is the optical property of a material having a refractive index that depends on the polarization and propagation direction of light. These optically anisotropic materials are said to be birefringent (or birefractive). The birefringence is often quantified as the maximum difference between refractive indices exhibited by the material. Crystals with non-cubic crystal structures are often birefringent, as are plastics under mechanical stress. Birefringence is responsible for the phenomenon of double refraction whereby a ray of light, when incident upon a birefringent material, is split by polarization into two rays taking slightly different paths. This effect was first described by Danish scientist Rasmus Bartholin in 1669, who observed it in calcite, a crystal having one of the strongest birefringences. In the 19th century Augustin-Jean Fresnel described the phenomenon in terms of polarization, understanding light as a wave with field components in transverse pola ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insulator (electricity)
An electrical insulator is a material in which electric current does not flow freely. The atoms of the insulator have tightly bound electrons which cannot readily move. Other materials—semiconductors and conductors—conduct electric current more easily. The property that distinguishes an insulator is its resistivity; insulators have higher resistivity than semiconductors or conductors. The most common examples are non-metals. A perfect insulator does not exist because even insulators contain small numbers of mobile charges ( charge carriers) which can carry current. In addition, all insulators become electrically conductive when a sufficiently large voltage is applied that the electric field tears electrons away from the atoms. This is known as the breakdown voltage of an insulator. Some materials such as glass, paper and PTFE, which have high resistivity, are very good electrical insulators. A much larger class of materials, even though they may have lower bulk resistivi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Seifertite
Seifertite is a silicate mineral with the formula SiO2 and is one of the densest polymorphs of silica. It has only been found in Martian and lunar meteorites, where it is presumably formed from either tridymite or cristobalite – other polymorphs of quartz – as a result of heating during the atmospheric re-entry and impact to the Earth, at an estimated minimal pressure of 35 GPa. It can also be produced in the laboratory by compressing cristobalite in a diamond anvil cell to pressures above 40 GPa. The mineral is named after Friedrich Seifert (born 1941), the founder of the Bayerisches Geoinstitut at University of Bayreuth, Germany, and is officially recognized by the International Mineralogical Association.Seifertite: A new natural very dense post-stishovite polymorph of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-quartz
The room-temperature form of quartz, α-quartz, undergoes a reversible change in crystal structure at 573 °C to form β-quartz. This phenomenon is called an inversion, and for the α to β quartz inversion is accompanied by a linear expansion of 0.45%. This inversion can lead to cracking of ceramic ware if cooling occurs too quickly through the inversion temperature. This is called ''dunting'', and the resultant faults as ''dunts''. To avoid such thermal shock faults, cooling rates not exceeding 50 °C/hour have been recommended. At 870 °C quartz ceases to be stable but, in the absence of fluxes, does not alter until a much higher temperature is reached, when, depending on the temperature and nature of the fluxes present, it is converted into the polymorphs of cristobalite and / or tridymite. These polymorphs also experience temperature-induced inversions. The inversion of cristobalite at 220 °C can be advantageous to achieve the ''cristobalite squeeze''. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tridymite
Tridymite is a high-temperature polymorph of silica and usually occurs as minute tabular white or colorless pseudo-hexagonal crystals, or scales, in cavities in felsic volcanic rocks. Its chemical formula is Si O2. Tridymite was first described in 1868 and the type location is in Hidalgo, Mexico. The name is from the Greek ''tridymos'' for ''triplet'' as tridymite commonly occurs as twinned crystal '' trillings'' (compound crystals comprising three twinned crystal components). Structure Tridymite can occur in seven crystalline forms. Two of the most common at standard pressure are known as α and β. The α-tridymite phase is favored at elevated temperatures (>870 °C) and it converts to β- cristobalite at 1470 °C. However, tridymite does usually not form from pure β-quartz, one needs to add trace amounts of certain compounds to achieve this. Otherwise the β-quartz-tridymite transition is skipped and β-quartz transitions directly to cristobalite at 1050 ° ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cristobalite
Cristobalite is a mineral polymorph of silica that is formed at very high temperatures. It has the same chemical formula as quartz, SiO2, but a distinct crystal structure. Both quartz and cristobalite are polymorphs with all the members of the quartz group, which also include coesite, tridymite and stishovite. It is named after Cerro San Cristóbal in Pachuca Municipality, Hidalgo, Mexico. It is used in dentistry as a component of alginate impression materials as well as for making models of teeth. Properties Metastability Cristobalite is stable only above 1470 °C, but can crystallize and persist metastably at lower temperatures. The persistence of cristobalite outside its thermodynamic stability range occurs because the transition from cristobalite to quartz or tridymite is "reconstructive", requiring the breaking up and reforming of the silica framework. These frameworks are composed of Si O4 tetrahedra in which every oxygen atom is shared with a neighbouring ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha Quartz
The room-temperature form of quartz, α-quartz, undergoes a reversible change in crystal structure at 573 °C to form β-quartz. This phenomenon is called an inversion, and for the α to β quartz inversion is accompanied by a linear expansion of 0.45%. This inversion can lead to cracking of ceramic ware if cooling occurs too quickly through the inversion temperature. This is called ''dunting'', and the resultant faults as ''dunts''. To avoid such thermal shock faults, cooling rates not exceeding 50 °C/hour have been recommended. At 870 °C quartz ceases to be stable but, in the absence of fluxes, does not alter until a much higher temperature is reached, when, depending on the temperature and nature of the fluxes present, it is converted into the polymorphs of cristobalite and / or tridymite. These polymorphs also experience temperature-induced inversions. The inversion of cristobalite at 220 °C can be advantageous to achieve the ''cristobalite squeeze''. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silicon–oxygen Bond
A silicon–oxygen bond ( bond) is a chemical bond between silicon and oxygen atoms that can be found in many inorganic and organic compounds. In a silicon–oxygen bond, electrons are shared unequally between the two atoms, with oxygen taking the larger share due to its greater electronegativity. This polarisation means Si–O bonds show characteristics of both covalent and ionic bonds. Compounds containing silicon–oxygen bonds include materials of major geological and industrial significance such as silica, silicate minerals and silicone polymers like polydimethylsiloxane. Bond polarity, length and strength On the Pauling electronegativity scale, silicon has an electronegativity of 1.90 and oxygen 3.44. The electronegativity difference between the elements is therefore 1.54. Because of this moderately large difference in electronegativities, the bond is polar but not fully ionic. Carbon has an electronegativity of 2.55 so carbon–oxygen bonds have an electronegativit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymorphism (materials Science)
In materials science, polymorphism describes the existence of a solid material in more than one form or crystal structure. Polymorphism is a form of isomerism. Any crystalline material can exhibit the phenomenon. Allotropy refers to polymorphism for chemical elements. Polymorphism is of practical relevance to pharmaceuticals, agrochemicals, pigments, dyestuffs, foods, and explosives. According to IUPAC, a polymorphic transition is "A reversible transition of a solid crystalline phase at a certain temperature and pressure (the inversion point) to another phase of the same chemical composition with a different crystal structure." According to McCrone, polymorphs are "different in crystal structure but identical in the liquid or vapor states." Materials with two polymorphs are called dimorphic, with three polymorphs, trimorphic, etc. Examples Many compounds exhibit polymorphism. It has been claimed that "every compound has different polymorphic forms, and that, in general, the nu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Double Bond Rule
In chemistry, the double bond rule states that elements with a principal quantum number greater than 2 for their valence electrons ( period 3 elements and higher) tend not to form multiple bonds (e.g. double bonds and triple bonds). The double bonds, when they exist, are often weak due to poor orbital overlap. Although such compounds are not intrinsically unstable, they instead tend to polymerize. An example is the rapid polymerization that occurs upon condensation of disulfur, the heavy analogue of . Numerous violations to the rule exist. Other meanings Another unrelated double bond rule exists that relates to the enhanced reactivity of sigma bonds attached to an atom adjacent to a double bond. In bromoalkenes, the C–Br bond is very stable, but in an allyl bromide, this bond is very reactive. Likewise, bromobenzenes are generally inert, whereas benzylic bromides are reactive. The first to observe the phenomenon was Conrad Laar Conrad Peter Laar (22 March 1853 – 1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral Coordination Geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1(−) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane () as well as its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group Td, but most tetrahedral molecules have lower symmetry. Tetrahedral molecules can be chiral. Tetrahedral bond angle The bond angle for a symmetric tetrahedral molecule such as CH4 may be calculated using the dot product of two vectors. As shown in the diagram, the molecule can be inscribed in a cube with the tetravalent atom (e.g. carbon) at the cube centre which is the origin of coordinates, O. The four monovalent atoms (e.g. hydrogens) are at four corners of the cube (A, B, C, D) chosen so that no two atoms are at adjacent corners linked by only one cube edge. If the edge length of the cube ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
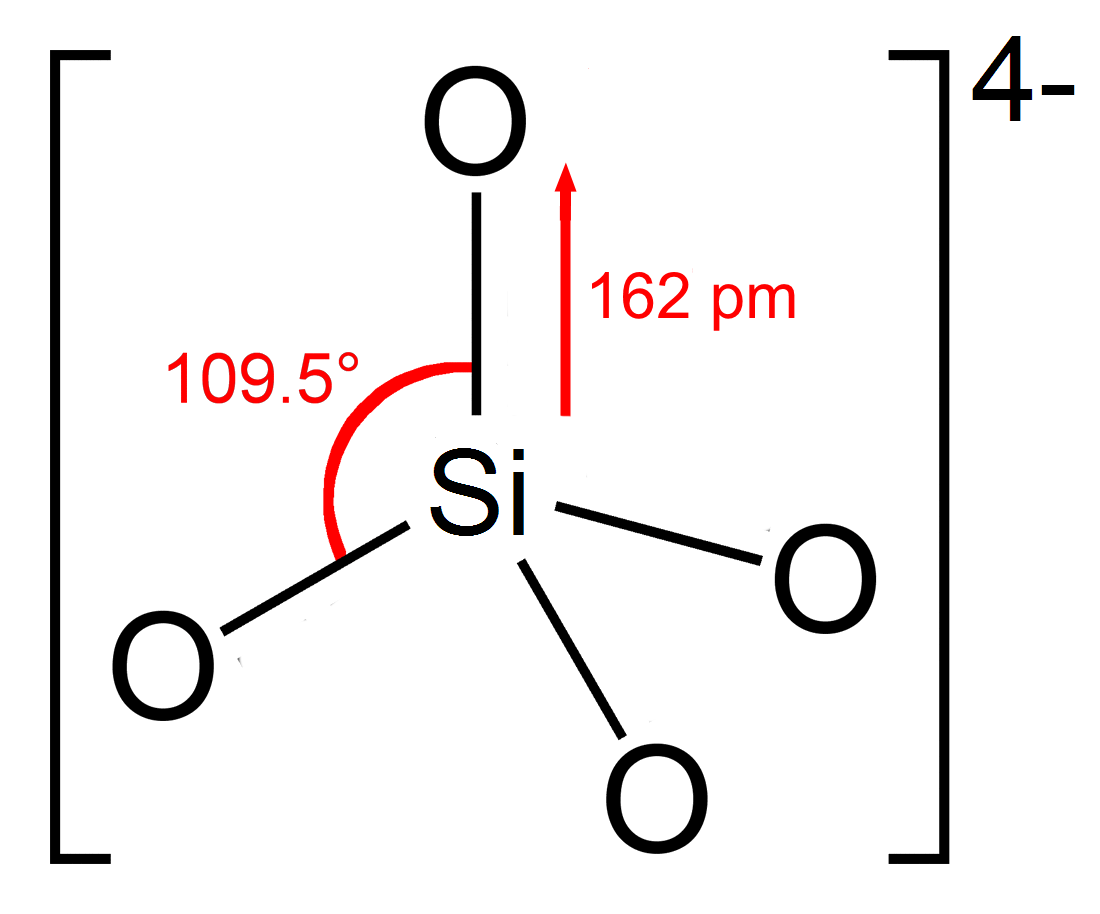
Silicate
In chemistry, a silicate is any member of a family of polyatomic anions consisting of silicon and oxygen, usually with the general formula , where . The family includes orthosilicate (), metasilicate (), and pyrosilicate (, ). The name is also used for any salt of such anions, such as sodium metasilicate; or any ester containing the corresponding chemical group, such as tetramethyl orthosilicate. The name "silicate" is sometimes extended to any anions containing silicon, even if they do not fit the general formula or contain other atoms besides oxygen; such as hexafluorosilicate .Most commonly, silicates are encountered as silicate minerals. For diverse manufacturing, technological, and artistic needs, silicates are versatile materials, both natural (such as granite, gravel, and garnet) and artificial (such as Portland cement, ceramics, glass, and waterglass). Structural principles In all silicates, silicon atom occupies the center of an idealized tetrahedro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |