|
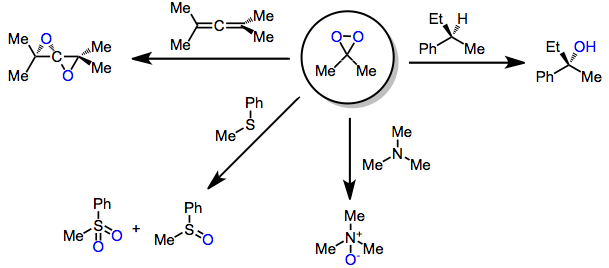
Shi Epoxidation
The Shi epoxidation is a chemical reaction described as the asymmetric epoxidation of alkenes with oxone (potassium peroxymonosulfate) and a fructose-derived catalyst (1). This reaction is thought to proceed via a dioxirane intermediate, generated from the catalyst ketone by oxone (potassium peroxymonosulfate). The addition of the sulfate group by the oxone facilitates the formation of the dioxirane by acting as a good leaving group during ring closure. It is notable for its use of a non-metal catalyst and represents an early example of organocatalysis. The reaction was first discovered by Yian Shi (史一安, pinyin: Shǐ Yī-ān) of Colorado State University in 1996. History Many attempts at the synthesis of an efficient non-metal catalyst were made before one was discovered. The problem with previous catalysts was the rapid decomposition/oxidation of the dioxirane intermediate and lack of electrophilicity of the reactive ketone. Aromatic ketones were proposed, and many su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridinium Chlorochromate
Pyridinium chlorochromate (PCC) is a yellow-orange salt (chemistry), salt with the chemical formula, formula [C5H5NH]+[CrO3Cl]−. It is a reagent in organic synthesis used primarily for organic redox reaction, oxidation of Alcohol (chemistry), alcohols to form carbonyls. A variety of related compounds are known with similar reactivity. PCC offers the advantage of the selective oxidation of alcohols to aldehydes or ketones, whereas many other reagents are less selective. Structure and preparation PCC consists of a pyridinium cation, [C5H5NH]+, and a tetrahedral chlorochromate anion, [CrO3Cl]−. Related salts are also known, such as 1-butylpyridinium chlorochromate, [C5H5N(C4H9)][CrO3Cl] and potassium chlorochromate. PCC is commercially available. Discovered by accident, the reagent was originally prepared via addition of pyridine into a cold solution of chromium trioxide in concentrated hydrochloric acid: :C5H5N + HCl + CrO3 → [C5H5NH][CrO3Cl] In one alternative method, form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Carbonate
Potassium carbonate is the inorganic compound with the formula K2 CO3. It is a white salt, which is soluble in water. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used in the production of soap and glass. History Potassium carbonate is the primary component of potash and the more refined pearl ash or salts of tartar. Historically, pearl ash was created by baking potash in a kiln to remove impurities. The fine, white powder remaining was the pearl ash. The first patent issued by the US Patent Office was awarded to Samuel Hopkins in 1790 for an improved method of making potash and pearl ash. In late 18th-century North America, before the development of baking powder, pearl ash was used as a leavening agent for quick breads. Production Potassium carbonate is prepared commercially by the reaction potassium hydroxide with carbon dioxide: : 2 KOH + CO2 → K2CO3 + H2O From the solution crystallizes the sesquihydrate K2CO3·H2O ("potash ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mechanism Of Dioxirane
Mechanism may refer to: *Mechanism (engineering), rigid bodies connected by joints in order to accomplish a desired force and/or motion transmission *Mechanism (biology), explaining how a feature is created *Mechanism (philosophy), a theory that all natural phenomena can be explained by physical causes *Mechanism (sociology), a theory that all social phenomena can be explained by the existence of a deterministic mechanism * "The Mechanism", song by Disclosure * ''The Mechanism'' (TV series), a Netflix TV series See also *Machine *Machine (mechanical) *Linkage (mechanical) *Mechanism design, the art of designing rules of a game to achieve a specific outcome *Mechanism of action, the means by which a drug exerts its biological effects *Defence mechanism, unconscious mechanisms aimed at reducing anxiety *Reaction mechanism, the sequence of reactions by which overall chemical change occurs *Antikythera mechanism, an ancient Greek analog computer *Theory of operation A theory of op ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidation With Dioxiranes
Oxidation with dioxiranes refers to the introduction of oxygen into organic molecules through the action of a dioxirane. Dioxiranes are well known for their oxidation of alkenes to epoxides; however, they are also able to oxidize other unsaturated functionality, heteroatoms, and alkane C-H bonds. Introduction Dioxiranes may be produced through the action of KHSO5 on carbonyl compounds. Because of their low-lying σ*O-O orbital, they are highly electrophilic oxidants and react with unsaturated functional groups, Y-H bonds (yielding oxygen insertion products), and heteroatoms. The most common dioxiranes employed for organic synthesis are dimethyldioxirane (DMD) and trifluoromethyl-methyldioxirane (TFD). The latter is effective for chemoselective oxidations of C-H and Si-H bonds. Although this class of reagents is most famous for the epoxidation of alkenes, dioxiranes have been used extensively for other kinds of oxidations as well. Mechanism and stereochemistry Prevailing mechanisms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxy Acid
A peroxy acid (often spelled as one word, peroxyacid, and sometimes called peracid) is an acid which contains an acidic –OOH group. The two main classes are those derived from conventional mineral acids, especially sulfuric acid, and the peroxy derivatives of organic carboxylic acids. They are generally strong oxidizers. Inorganic peroxy acids Peroxymonosulfuric acid (Caro's acid) is probably the most important inorganic peracid, at least in terms of its production scale. It is used for the bleaching of pulp and for the detoxification of cyanide in the mining industry. It is produced by treating sulfuric acid with hydrogen peroxide. Peroxymonophosphoric acid (H3PO5) is prepared similarly. Organic peracids Several organic peroxyacids are commercially useful. They can be prepared in several ways. Most commonly, peracids are generated by treating the corresponding carboxylic acid with hydrogen peroxide: :RCO2H + H2O2 RCO3H + H2O A related reaction involves treatment o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
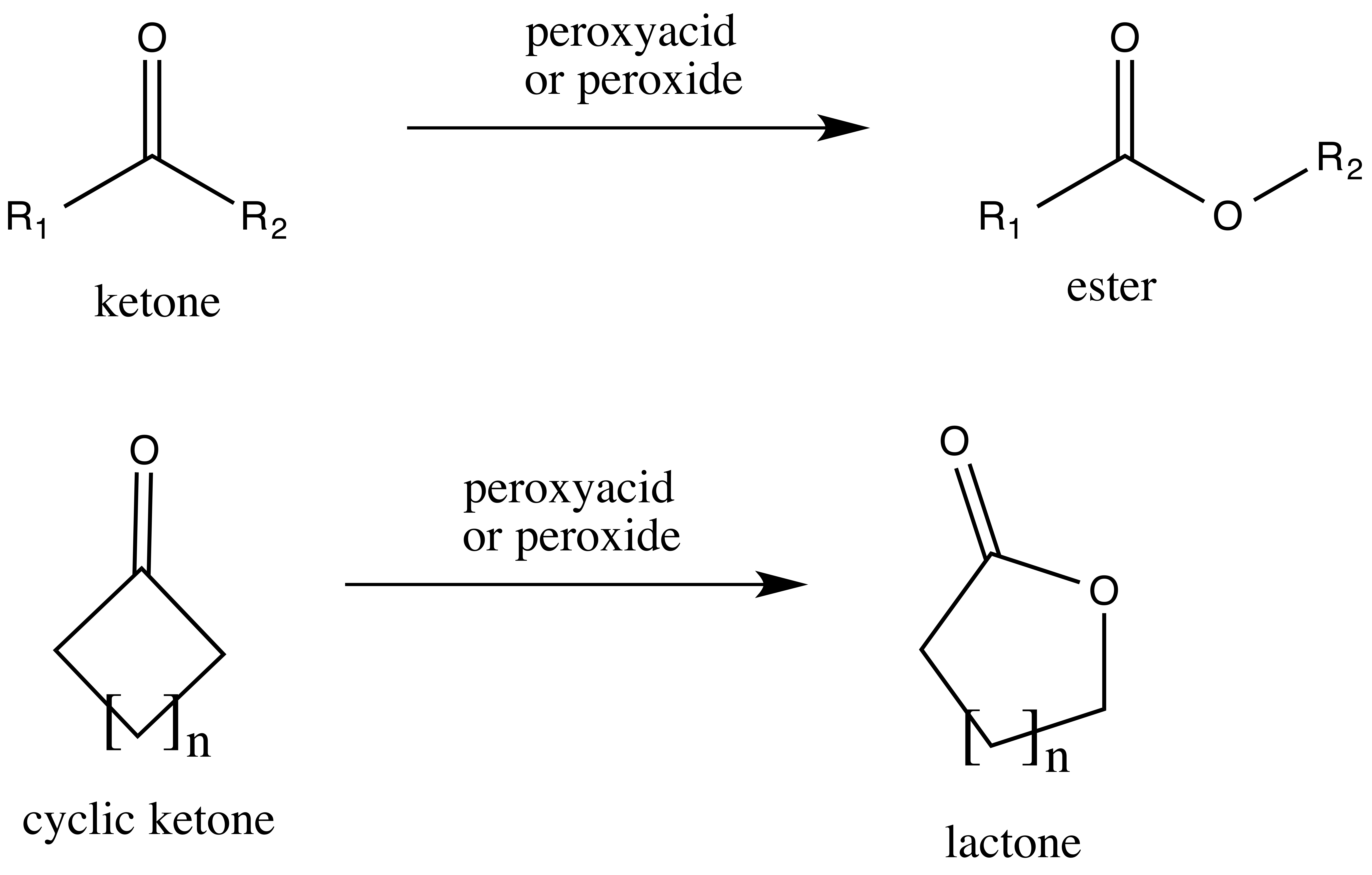
Baeyer–Villiger Oxidation
The Baeyer–Villiger oxidation is an organic reaction that forms an ester from a ketone or a lactone from a cyclic ketone, using peroxyacids or peroxides as the oxidant. The reaction is named after Adolf von Baeyer and Victor Villiger who first reported the reaction in 1899. Reaction mechanism In the first step of the reaction mechanism, the peroxyacid protonates the oxygen of the carbonyl group. This makes the carbonyl group more susceptible to be attacked by the peroxyacid. Next, the peroxyacid attacks the carbon of the carbonyl group forming what is known as the Criegee intermediate. Through a concerted mechanism, one of the substituents on the ketone group migrates to the oxygen of the peroxide group while a carboxylic acid leaves. This migration step is thought to be the rate determining step. Finally, deprotonation of the oxocarbenium ion produces the ester. The products of the Baeyer–Villiger oxidation are believed to be controlled through both primary and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Baldwin's Rules
Baldwin's rules in organic chemistry are a series of guidelines outlining the relative favorabilities of ring closure reactions in alicyclic compounds. They were first proposed by Jack Baldwin in 1976. Baldwin's rules discuss the relative rates of ring closures of these various types. These terms are not meant to describe the absolute probability that a reaction will or will not take place, rather they are used in a relative sense. A reaction that is disfavoured (slow) does not have a rate that is able to compete effectively with an alternative reaction that is favoured (fast). However, the disfavoured product may be observed, if no alternate reactions are more favoured. The rules classify ring closures in three ways: *the number of atoms in the newly formed ring *into ''exo'' and ''endo'' ring closures, depending whether the bond broken during the ring closure is inside (''endo'') or outside (''exo'') the ring that is being formed *into ''tet'', ''trig'' and ''dig'' geometry of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Criegee Biradical
A Criegee intermediate (also called a Criegee zwitterion or Criegee biradical) is a carbonyl oxide with two charge centres. These chemicals may react with sulfur dioxide and nitrogen oxides in the earth's atmosphere, and are implicated in the formation of aerosols, which are an important factor in controlling global climate. Criegee intermediates are also an important source of OH (hydroxyl radicals). OH radicals are the most important oxidant in the troposphere, and are important in controlling air quality and pollution. The formation of this sort of structure was first postulated in the 1950s by Rudolf Criegee, for whom it is named. It was not until 2012 that direct detection of such chemicals was reported. Infrared spectroscopy suggests the electronic structure has a substantially zwitterionic character rather than the biradical character that had previously been proposed. Formation Criegee intermediates are formed by the gas-phase reactions of alkenes and ozone in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleophilic Addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions differ from electrophilic additions in that the former reactions involve the group to which atoms are added accepting electron pairs, whereas the latter reactions involve the group donating electron pairs. Addition to carbon–heteroatom double bonds Nucleophilic addition reactions of nucleophiles with electrophilic double or triple bond (π bonds) create a new carbon center with two additional single, or σ, bonds.March Jerry; (1985). Advanced Organic Chemistry reactions, mechanisms and structure (3rd ed.). New York: John Wiley & Sons, inc. Addition of a nucleophile to carbon–heteroatom double or triple bonds such as >C=O or -C≡N show great variety. These types of bonds are polar (have a large difference in electronegativity betwe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |