Shi epoxidation on:
[Wikipedia]
[Google]
[Amazon]
 The Shi epoxidation is a
The Shi epoxidation is a
 Decomposition of reagents is bimolecular (
Decomposition of reagents is bimolecular (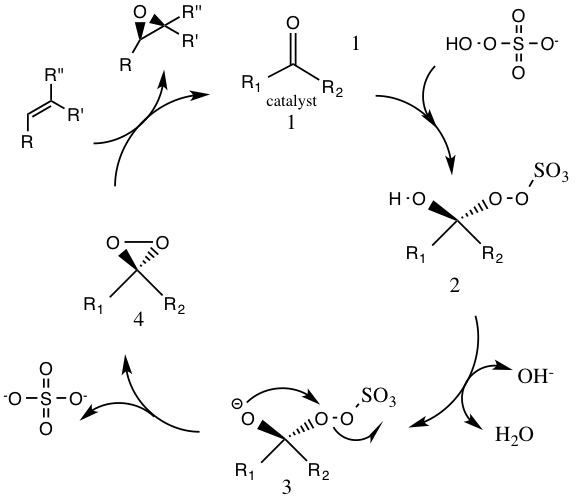 The first step in the catalytic cycle reaction is the
The first step in the catalytic cycle reaction is the



 The previously mentioned configurations are favored over the transition states of the opposing enantiomers because of unfavorable steric interactions between the R-alkyl groups (see below) and the ether-alkyl functional groups of the catalyst ring.
The previously mentioned configurations are favored over the transition states of the opposing enantiomers because of unfavorable steric interactions between the R-alkyl groups (see below) and the ether-alkyl functional groups of the catalyst ring.
 The enantiomeric success of this epoxidation is relatively high compared to metal catalysts, and generally results in a high enantiomeric excess exceeding 80 percent.
The enantiomeric success of this epoxidation is relatively high compared to metal catalysts, and generally results in a high enantiomeric excess exceeding 80 percent.

# Denmark, Wu, et al. "The Development of Chiral, Non-Racemic Dioxiranes for the Catalytic, Enantioselective Epoxidation of Alkenes". (13, April 1999)
# Frohn, Shi, Tu, Wang, Zhang, et al. "An Efficient Asymmetric Epoxidation Method". (8 July 1997)
# Shi, Wang, et al. "A New Type of Ketone Catalyst for Asymmetric Epoxidation". (12 September 1997).
 The Shi epoxidation is a
The Shi epoxidation is a chemical reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the pos ...
described as the asymmetric epoxidation
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for ...
of alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s with oxone
Potassium peroxymonosulfate is widely used as an oxidizing agent. It is the potassium salt of peroxymonosulfuric acid. Usually potassium peroxymonosulfate refers to the triple salt known as oxone.
The standard electrode potential for potassium p ...
(potassium peroxymonosulfate) and a fructose
Fructose, or fruit sugar, is a Ketose, ketonic monosaccharide, simple sugar found in many plants, where it is often bonded to glucose to form the disaccharide sucrose. It is one of the three dietary monosaccharides, along with glucose and galacto ...
-derived catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
(1). This reaction is thought to proceed via a dioxirane
In chemistry, dioxirane is a compound with formula , whose molecule consists of a ring with one carbon and two oxygen atoms, and two hydrogen atoms attached to the carbon. It is a heterocyclic compound, the smallest cyclic organic peroxide.
Th ...
intermediate, generated from the catalyst ketone by oxone
Potassium peroxymonosulfate is widely used as an oxidizing agent. It is the potassium salt of peroxymonosulfuric acid. Usually potassium peroxymonosulfate refers to the triple salt known as oxone.
The standard electrode potential for potassium p ...
(potassium peroxymonosulfate). The addition of the sulfate group by the oxone facilitates the formation of the dioxirane by acting as a good leaving group during ring closure. It is notable for its use of a non-metal catalyst and represents an early example of organocatalysis
In organic chemistry, organocatalysis is a form of catalysis in which the rate of a chemical reaction is increased by an organic catalyst. This "organocatalyst" consists of carbon, hydrogen, sulfur and other nonmetal elements found in organic com ...
. The reaction was first discovered by Yian Shi (史一安, pinyin
Hanyu Pinyin (), often shortened to just pinyin, is the official romanization system for Standard Mandarin Chinese in China, and to some extent, in Singapore and Malaysia. It is often used to teach Mandarin, normally written in Chinese for ...
: Shǐ Yī-ān) of Colorado State University in 1996.
History
Many attempts at the synthesis of an efficient non-metal catalyst were made before one was discovered. The problem with previous catalysts was the rapid decomposition/oxidation of the dioxirane intermediate and lack of electrophilicity of the reactive ketone. Aromatic ketones were proposed, and many subsequent variations of oxoammonium salts were used, but were ineffective in promoting epoxidation because of the oxidative instability of the amide groups and high flexibility of the seven-membered rings. Enantioselectivity of these early catalysts were also lowered because of large distances between the asymmetric subunits and reaction centers, yielding less than 10 percent inenantiomeric excess
In stereochemistry, enantiomeric excess (ee) is a measurement of purity used for chiral substances. It reflects the degree to which a sample contains one enantiomer in greater amounts than the other. A racemic mixture has an ee of 0%, while a sin ...
.
The catalyst discovered by Yian Shi's group in 1996 was derived from D-fructose, and has a stereogenic center close to the reacting center (ketone)- the rigid six-membered ring structure of the catalyst and adjacent quaternary ring group minimizes epimerization of this stereocenter. Oxidation by the active dioxirane catalyst takes place from the si-face, due to steric hindrance of the opposing re-face. This catalyst functions efficiently as an asymmetric catalyst for unfunctionalized trans-olefins.
Dioxirane catalyst formation
Under normal pH conditions, an excess of 3 stoichiometric amounts of ketone catalyst are needed due to a high rate of decomposition. At basic pH conditions greater than 10 (pH 10.5) substoichiometric amounts (0.2–0.3) are needed for epoxidations, lowering the decomposition of reagents by disfavoring the Baeyer-Villiger side reaction. Higher temperatures result in further decomposition; thus a low temperature of zero degrees Celsius is used. Decomposition of reagents is bimolecular (
Decomposition of reagents is bimolecular (second-order reaction
In chemistry, the rate law or rate equation for a reaction is an equation that links the initial or forward reaction rate with the concentrations or pressures of the reactants and constant parameters (normally rate coefficients and partial react ...
rate), so low amounts of oxone and catalyst are used.
The reaction is mediated by a D-fructose derived catalyst, which produces the (R,R) enantiomer of the resulting epoxide. Solubilities of olefin organic substrate and oxidant (oxone) differ, and thus a biphasic
Biphasic, meaning having two phases, may refer to:
* Phase (matter)
In the physical sciences, a phase is a region of space (a thermodynamic system), throughout which all physical properties of a material are essentially uniform. Examples ...
medium is needed. The generation of the active catalyst species takes place in the aqueous layer, and is shuttled to the organic layer with the reactants by tetrabutylammonium sulfate. The ketone catalyst is continuously regenerated in a catalytic cycle, and thus can catalyze the epoxidation
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for ...
in small amounts.
 The first step in the catalytic cycle reaction is the
The first step in the catalytic cycle reaction is the nucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions di ...
reaction of the oxone with the ketone group on the catalyst (intermediate 1). This forms the reactive intermediate number 2 species, the Criegee intermediate
A Criegee intermediate (also called a Criegee zwitterion or Criegee biradical) is a carbonyl oxide with two charge centres. These chemicals may react with sulfur dioxide and nitrogen oxides in the earth's atmosphere, and are implicated in the f ...
that can potentially lead to unwanted side reactions, such as the Baeyer-Villiger reaction (see below). The generation of intermediate species number 3 occurs under basic conditions, with a removal of the hydrogen from the hydroxy group to form a nucleophilic oxygen anion. The sulfate group facilitates the subsequent formation of the dioxirane, intermediate species number 4, by acting as a good leaving group during the 3-exo-tet cyclization. The activated dioxirane catalytic species then transfers an oxygen atom to the alkene, leading to a regeneration of the original catalyst.Side reactions
A potential side reaction that may occur is the Baeyer-Villiger reaction of intermediate 2, where there is a rearrangement of theperoxy
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable.
The most common peroxide is hydrogen ...
group that results in the formation of the relative ester. The extent of this side reaction declines with the rise of pH, and increases the nucleophilicity of the oxone, making basic conditions favorable for the overall epoxidation and reactivity of the catalytic species.

Epoxidation mechanisms
The oxygen from the dioxirane group generated on the organic catalyst is transferred to the alkene, in what is thought to be a concerted mechanism, although the presence of an oxygen anion intermediate through an Sn2 mechanism may transpire.
Preparation of D-fructose derivative
The catalyst is formed by reaction with acetone under basic conditions, with the hydroxyl groups of the fructose ring acting as nucleophiles, their nucleophilicity increased by the basic conditions created bypotassium carbonate
Potassium carbonate is the inorganic compound with the formula K2 CO3. It is a white salt, which is soluble in water. It is deliquescent, often appearing as a damp or wet solid. Potassium carbonate is mainly used in the production of soap and gl ...
. The electron withdrawing substituents (alpha-ether groups) encourage the formation of the ketone from the oxidizing agent pyridinium chlorochromate
Pyridinium chlorochromate (PCC) is a yellow-orange salt (chemistry), salt with the chemical formula, formula 5H5NH rO3Cl��. It is a reagent in organic synthesis used primarily for organic redox reaction, oxidation of Alcohol (chemistry), al ...
by increasing the electrophilicity of the carbonyl carbon, via a stabilizing delocalization of the forming π C-C bonds into the σ* C-O bonds of the adjacent ethers.

Transition states and enantiomeric selectivity
There are two proposed transition states, whose geometries are speculated and not corroborated by experimental evidence, but are attributed tostereoelectronic effect
In chemistry, primarily organic and computational chemistry, a stereoelectronic effectAlabugin, I. V. Stereoelectronic Effects: the Bridge between Structure and Reactivity. John Wiley & Sons Ltd, Chichester, UK, 2016. http://eu.wiley.com/WileyCDA/W ...
s. The spiro transition state is favored over the planar due to the non-bonding orbitals of the superior oxygen donating into the π* anti-bonding C-C orbitals of the reacting alkene, providing a stabilizing delocalization of electrons.
Donation of these electrons into the forming C-O σ bonds of the epoxide bonds also encourages the formation of the
spiro-product (the geometry of the product is aligned as well). The planar configuration is disfavored due to lack of pi-backbonding and steric hindrance of the alkyl groups with large alkyl functional groups of the catalytic ring.
 The previously mentioned configurations are favored over the transition states of the opposing enantiomers because of unfavorable steric interactions between the R-alkyl groups (see below) and the ether-alkyl functional groups of the catalyst ring.
The previously mentioned configurations are favored over the transition states of the opposing enantiomers because of unfavorable steric interactions between the R-alkyl groups (see below) and the ether-alkyl functional groups of the catalyst ring.
 The enantiomeric success of this epoxidation is relatively high compared to metal catalysts, and generally results in a high enantiomeric excess exceeding 80 percent.
The enantiomeric success of this epoxidation is relatively high compared to metal catalysts, and generally results in a high enantiomeric excess exceeding 80 percent.
Reaction yield and stereoselectivity
This procedure generates epoxides with highenantiomeric excess
In stereochemistry, enantiomeric excess (ee) is a measurement of purity used for chiral substances. It reflects the degree to which a sample contains one enantiomer in greater amounts than the other. A racemic mixture has an ee of 0%, while a sin ...
es from trans-disubstituted alkenes and trisubstituted alkenes. Cis-disubstituted alkenes and styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
s are asymmetrically epoxidized using a similar catalyst. Generation of (R,R) epoxides from corresponding alkenes increases in stereoselectivity with increased steric bulk of substituent R groups (especially in trans-olefins).

References
# ''An Efficient Catalytic Asymmetric Epoxidation Method'' Zhi-Xian Wang, Yong Tu, Michael Frohn, Jian-Rong Zhang, and Yian Shi ''J. Am. Chem. Soc.
The ''Journal of the American Chemical Society'' is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytic ...
'' 1997, ''119(46)'', 11224-11235. ()
# Frohn, M.; Shi, Y. ''Synthesis'' 2000, ''14'', 1979-2000 . (Review)
# Tian, H.; She, X.; Shu, L.; Yu, H.; Shi, Y. ''J. Am. Chem. Soc.
The ''Journal of the American Chemical Society'' is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ''Journal of Analytic ...
'' 2000, ''122'', 11551-11552. ()
# Tian, H.; She, X.; Xu, J.; Shi, Y. '' Org. Lett.'' 2001, ''3'', 1929-1931. ()
# Shi Epoxidation See also
*Sharpless epoxidation
The Sharpless epoxidation reaction is an enantioselective chemical reaction to prepare 2,3-epoxyalcohols from primary and secondary allylic alcohols. The oxidizing agent is ''tert''-butyl hydroperoxide. The method relies on a catalyst formed fro ...
{{DEFAULTSORT:Shi Epoxidation
Epoxidation reactions
Oxygen heterocycle forming reactions
Organic oxidation reactions
Name reactions