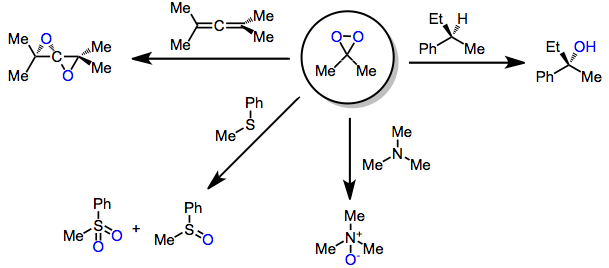
|
Oxidation With Dioxiranes
Oxidation with dioxiranes refers to the introduction of oxygen into organic molecules through the action of a dioxirane. Dioxiranes are well known for their oxidation of alkenes to epoxides; however, they are also able to oxidize other unsaturated functionality, heteroatoms, and alkane C-H bonds. Introduction Dioxiranes may be produced through the action of KHSO5 on carbonyl compounds. Because of their low-lying σ*O-O orbital, they are highly electrophilic oxidants and react with unsaturated functional groups, Y-H bonds (yielding oxygen insertion products), and heteroatoms. The most common dioxiranes employed for organic synthesis are dimethyldioxirane (DMD) and trifluoromethyl-methyldioxirane (TFD). The latter is effective for chemoselective oxidations of C-H and Si-H bonds. Although this class of reagents is most famous for the epoxidation of alkenes, dioxiranes have been used extensively for other kinds of oxidations as well. Mechanism and stereochemistry Prevailing mechanisms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dioxirane
In chemistry, dioxirane is a compound with formula , whose molecule consists of a ring with one carbon and two oxygen atoms, and two hydrogen atoms attached to the carbon. It is a heterocyclic compound, the smallest cyclic organic peroxide. The compound itself is highly unstable and has never been observed at room temperature. Derivatives in which the hydrogens are replaced by other functional groups are called dioxiranes, and may be more stable. Some of them, such as dimethyldioxirane (DMDO) and the more reactive methyl(trifluoromethyl)dioxirane, are used in organic synthesis as oxidizing reagents, most notably as the key catalytic intermediate in the Shi epoxidation reaction. Difluorodioxirane, which boils at about –80 to –90 °C, is one of the very few dioxirane derivatives that is stable in pure form at room temperature. Synthesis Dioxirane is highly unstable and the majority of studies of it have been computational; it has been detected during the low tempera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adamantane
Adamantane is an organic compound with a formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the most stable isomer of C10H16. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This similarity led to the name ''adamantane'', which is derived from the Greek ''adamantinos'' (relating to steel or diamond). It is a white solid with a camphor-like odor. It is the simplest diamondoid. The discovery of adamantane in petroleum in 1933 launched a new field of chemistry dedicated to the synthesis and properties of polyhedral organic compounds. Adamantane derivatives have found practical application as drugs, polymeric materials, and thermally stable lubricants. History and synthesis In 1924, H. Decker suggested the existence of adamantane, which he called decaterpene. The first attempted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trifluoroacetone
Trifluoroacetone (1,1,1-trifluoroacetone) is an organofluorine compound with the chemical formula CF3C(O)CH3. The compound is a colorless liquid with chloroform-like odour. Preparation, reactions, uses Trifluoroacetone is produced by decarboxylation of trifluoroacetoacetic acid: :CF3C(O)CH2CO2H → CF3C(O)CH3 + CO2 The acetoacetic acid in turn is obtained via condensation of acetate and trifluoroacetate esters. Trifluoroacetone has been examined as oxidizing agent in Oppenauer oxidation, in which case hydroxyl groups of secondary alcohols can be oxidized in the presence of hydroxy groups of primary alcohols. Trifluoracetone is also used in a synthesis of 2-trifluoromethyl-7-azaindoles starting with 2,6-dihalopyridines. The derived chiral imine is used to prepare enantiopure α-trifluoromethyl alanines and diamines by a Strecker reaction followed by either nitrile hydrolysis or reduction. See also *Hexafluoroacetone Hexafluoroacetone (HFA) is a chemical compound with the formu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peroxides
In chemistry, peroxides are a group of compounds with the structure , where R = any element. The group in a peroxide is called the peroxide group or peroxo group. The nomenclature is somewhat variable. The most common peroxide is hydrogen peroxide (), colloquially known simply as "peroxide". It is marketed as solutions in water at various concentrations. Many organic peroxides are known as well. In addition to hydrogen peroxide, some other major classes of peroxides are: * Peroxy acids, the peroxy derivatives of many familiar acids, examples being peroxymonosulfuric acid and peracetic acid, and their salts, one example of which is potassium peroxydisulfate. * Main group peroxides, compounds with the linkage (E = main group element). * Metal peroxides, examples being barium peroxide (), sodium peroxide () and zinc peroxide (). * Organic peroxide In organic chemistry, organic peroxides are organic compounds containing the peroxide functional group (). If the R′ is h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyltrioxorhenium
Methylrhenium trioxide, also known as methyltrioxorhenium(VII), is an organometallic compound with the formula CH3ReO3. It is a volatile, colourless solid that has been used as a catalyst in some laboratory experiments. In this compound, rhenium has a tetrahedral coordination geometry with one methyl and three oxo ligands. The oxidation state of rhenium is +7. Synthesis Methylrhenium trioxide is commercially available. It can be prepared by many routes, a typical method is the reaction of rhenium heptoxide and tetramethyltin: :Re2O7 + (CH3)4Sn → CH3ReO3 + (CH3)3SnOReO3 Analogous alkyl and aryl derivatives are known. Compounds of the type RReO3 are Lewis acids, forming both 1:1 and 1:2 adducts with halides and amines. Uses Methylrhenium trioxide serves as a heterogeneous catalyst for a variety of transformations. Supported on alumina/silica, it catalyzes olefin metathesis at 25 °C. In solution, MTO catalyses for the oxidations with hydrogen peroxide. Termina ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscous than water. It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use, and in higher concentrations for industrial use. Concentrated hydrogen peroxide, or " high-test peroxide", decomposes explosively when heated and has been used as a propellant in rocketry. Hydrogen peroxide is a reactive oxygen species and the simplest peroxide, a compound having an oxygen–oxygen single bond. It decomposes slowly when exposed to light, and rapidly in the presence of organic or reactive compounds. It is typically stored with a stabilizer in a weakly acidic solution in a dark bottle to block light. Hydrogen peroxide is found in biological systems including the human body. Enzymes that use or decompose hydrogen peroxide are classified as peroxidases. Properties The boiling poi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Singlet Oxygen
Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemical with the formula O=O (also written as or ), which is in a quantum state where all electrons are spin paired. It is kinetically unstable at ambient temperature, but the rate of decay is slow. The lowest excited state of the diatomic oxygen molecule is a singlet state. It is a gas with physical properties differing only subtly from those of the more prevalent triplet ground state of O2. In terms of its chemical reactivity, however, singlet oxygen is far more reactive toward organic compounds. It is responsible for the photodegradation of many materials but can be put to constructive use in preparative organic chemistry and photodynamic therapy. Trace amounts of singlet oxygen are found in the upper atmosphere and also in polluted urban atmospheres where it contributes to the formation of lung-damaging nitrogen dioxide. It often appears and coexists confounded in environments ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxaziridine
An oxaziridine is an organic molecule that features a three-membered heterocycle containing oxygen, nitrogen, and carbon. In their largest application, oxaziridines are intermediates in the industrial production of hydrazine. Oxaziridine derivatives are also used as specialized reagents in organic chemistry for a variety of oxidations, including alpha hydroxylation of enolates, epoxidation and aziridination of olefins, and other heteroatom transfer reactions. Oxaziridines also serve as precursors to amides and participate in +2cycloadditions with various heterocumulenes to form substituted five-membered heterocycles. Chiral oxaziridine derivatives effect asymmetric oxygen transfer to prochiral enolates as well as other substrates. Some oxaziridines also have the property of a high barrier to inversion of the nitrogen, allowing for the possibility of chirality at the nitrogen center. History Oxaziridine derivatives were first reported in the mid-1950s by Emmons and subsequ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |