|
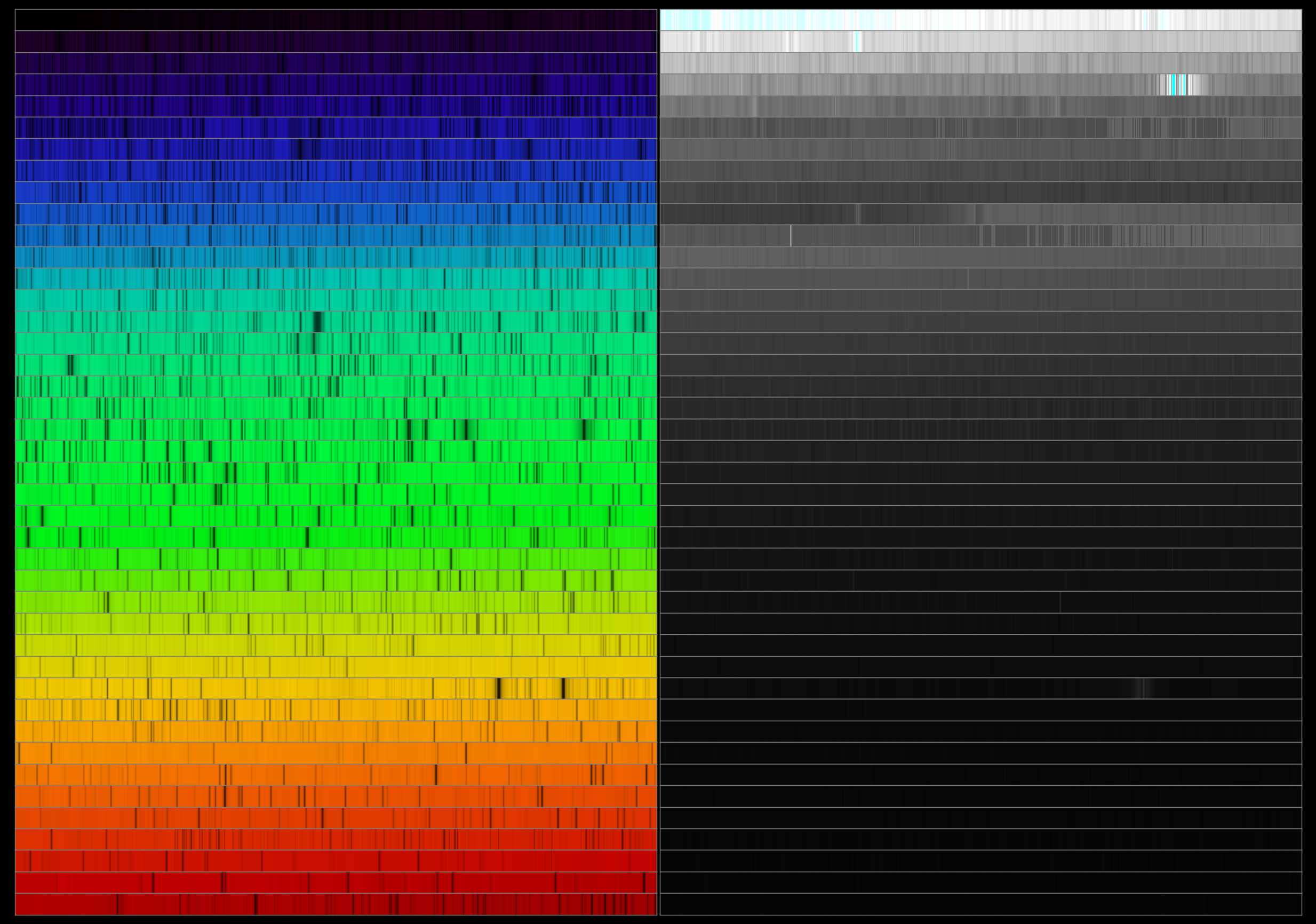
Second Solar Spectrum
The second solar spectrum is an electromagnetic spectrum of the Sun that shows the degree of linear polarization. The term was coined by V. V. Ivanov in 1991. The polarization is at a maximum close to the limb (edge) of the Sun, thus the best place to observe such a spectrum is from just inside the limb. It is also possible to get polarized light from outside the limb, but since this is much dimmer compared to the disk of the Sun, it is very easily polluted by scattered light. The second solar spectrum differs significantly from the solar spectrum determined by the intensity of light. Large effects come around the Ca II K and H line. These have broad effects 200 Å wide and show a sign reversal at their centers. Molecular lines with stronger polarization than the background due to MgH and C2 are common. Rare-earth elements stand out far more than expected from the intensity spectrum. Other odd lines include Li I at 6708 Å which has 0.005% more polarization at its pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Second Solar Spectrum
The second solar spectrum is an electromagnetic spectrum of the Sun that shows the degree of linear polarization. The term was coined by V. V. Ivanov in 1991. The polarization is at a maximum close to the limb (edge) of the Sun, thus the best place to observe such a spectrum is from just inside the limb. It is also possible to get polarized light from outside the limb, but since this is much dimmer compared to the disk of the Sun, it is very easily polluted by scattered light. The second solar spectrum differs significantly from the solar spectrum determined by the intensity of light. Large effects come around the Ca II K and H line. These have broad effects 200 Å wide and show a sign reversal at their centers. Molecular lines with stronger polarization than the background due to MgH and C2 are common. Rare-earth elements stand out far more than expected from the intensity spectrum. Other odd lines include Li I at 6708 Å which has 0.005% more polarization at its pe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electromagnetic Spectrum
The electromagnetic spectrum is the range of frequencies (the spectrum) of electromagnetic radiation and their respective wavelengths and photon energies. The electromagnetic spectrum covers electromagnetic waves with frequencies ranging from below one hertz to above 1025 hertz, corresponding to wavelengths from thousands of kilometers down to a fraction of the size of an atomic nucleus. This frequency range is divided into separate bands, and the electromagnetic waves within each frequency band are called by different names; beginning at the low frequency (long wavelength) end of the spectrum these are: radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, and gamma rays at the high-frequency (short wavelength) end. The electromagnetic waves in each of these bands have different characteristics, such as how they are produced, how they interact with matter, and their practical applications. There is no known limit for long and short wavelengths. Extreme ultr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linear Polarization
In electrodynamics, linear polarization or plane polarization of electromagnetic radiation is a confinement of the electric field vector or magnetic field vector to a given plane along the direction of propagation. The term ''linear polarization'' (French: ''polarisation rectiligne'') was coined by Augustin-Jean Fresnel in 1822.A. Fresnel, "Mémoire sur la double réfraction que les rayons lumineux éprouvent en traversant les aiguilles de cristal de roche suivant les directions parallèles à l'axe", read 9 December 1822; printed in H. de Senarmont, E. Verdet, and L. Fresnel (eds.), ''Oeuvres complètes d'Augustin Fresnel'', vol. 1 (1866), pp.731–51; translated as "Memoir on the double refraction that light rays undergo in traversing the needles of quartz in the directions parallel to the axis", , 2021 (open access); §9. See '' polarization'' and ''plane of polarization'' for more information. The orientation of a linearly polarized electromagn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Monohydride
Magnesium monohydride is a molecular gas with formula MgH that exists at high temperatures, such as the atmospheres of the Sun and stars. It was originally known as magnesium hydride, although that name is now more commonly used when referring to the similar chemical magnesium dihydride. History George Downing Liveing and James Dewar are claimed to be the first to make and observe a spectral line from MgH in 1878. However they did not realise what the substance was. Formation A laser can evaporate magnesium metal to form atoms that react with molecular hydrogen gas to form MgH and other magnesium hydrides. An electric discharge through hydrogen gas at low pressure (20 pascals) containing pieces of magnesium can produce MgH. Thermally produced hydrogen atoms and magnesium vapour can react and condense in a solid argon matrix. This process does not work with solid neon, probably due to the formation of instead. A simple way to produce some MgH is to burn magnesium in a bunsen b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dicarbon
Diatomic carbon (systematically named dicarbon and 1λ2,2λ2-ethene), is a green, gaseous inorganic chemical with the chemical formula C=C (also written 2or C2). It is kinetically unstable at ambient temperature and pressure, being removed through autopolymerisation. It occurs in carbon vapor, for example in electric arcs; in comets, stellar atmospheres, and the interstellar medium; and in blue hydrocarbon flames. Diatomic carbon is the second simplest form of carbon after atomic carbon, and is an intermediate participator in the genesis of fullerenes. Properties C2 is a component of carbon vapor. One paper estimates that carbon vapor is around 28% diatomic, but theoretically this depends on the temperature and pressure. Electromagnetic properties The electrons in diatomic carbon are distributed among the molecular orbitals according to the Aufbau principle to produce unique quantum states, with corresponding energy levels. The state with the lowest energy level, or ground ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rare-earth Element
The rare-earth elements (REE), also called the rare-earth metals or (in context) rare-earth oxides or sometimes the lanthanides (yttrium and scandium are usually included as rare earths), are a set of 17 nearly-indistinguishable lustrous silvery-white soft heavy metals. Compounds containing rare earths have diverse applications in electrical and electronic components, lasers, glass, magnetic materials, and industrial processes. Scandium and yttrium are considered rare-earth elements because they tend to occur in the same ore deposits as the lanthanides and exhibit similar chemical properties, but have different electronic and magnetic properties. These metals tarnish slowly in air at room temperature and react slowly with cold water to form hydroxides, liberating hydrogen. They react with steam to form oxides, and at elevated temperature (400°C) ignite spontaneously. These elements and their compounds have no biological function other than in several specialized enzymes, s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperfine Structure
In atomic physics, hyperfine structure is defined by small shifts in otherwise degenerate energy levels and the resulting splittings in those energy levels of atoms, molecules, and ions, due to electromagnetic multipole interaction between the nucleus and electron clouds. In atoms, hyperfine structure arises from the energy of the nuclear magnetic dipole moment interacting with the magnetic field generated by the electrons and the energy of the nuclear electric quadrupole moment in the electric field gradient due to the distribution of charge within the atom. Molecular hyperfine structure is generally dominated by these two effects, but also includes the energy associated with the interaction between the magnetic moments associated with different magnetic nuclei in a molecule, as well as between the nuclear magnetic moments and the magnetic field generated by the rotation of the molecule. Hyperfine structure contrasts with '' fine structure'', which results from the interaction b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rayleigh Scattering
Rayleigh scattering ( ), named after the 19th-century British physicist Lord Rayleigh (John William Strutt), is the predominantly elastic scattering of light or other electromagnetic radiation by particles much smaller than the wavelength of the radiation. For light frequencies well below the resonance frequency of the scattering particle (normal dispersion regime), the amount of scattering is inversely proportional to the fourth power of the wavelength. Rayleigh scattering results from the electric polarizability of the particles. The oscillating electric field of a light wave acts on the charges within a particle, causing them to move at the same frequency. The particle, therefore, becomes a small radiating dipole whose radiation we see as scattered light. The particles may be individual atoms or molecules; it can occur when light travels through transparent solids and liquids, but is most prominently seen in gases. Rayleigh scattering of sunlight in Earth's atmosphere causes d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron's mass is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum ( spin) of a half-integer value, expressed in units of the reduced Planck constant, . Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all elementary particles, electrons exhibit properties of both particles and waves: They can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a longer de Broglie wavele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of nitrogen, etc. For inorganic chemists, hydrides refer to compounds and ions in which hydrogen is covalently attached to a less electronegative element. In such cases, the H centre has nucleophilic character, which contrasts with the protic character of acids. The hydride anion is very rarely observed. Almost all of the elements form binary compounds with hydrogen, the exceptions being He, Ne, Ar, Kr, Pm, Os, Ir, Rn, Fr, and Ra. Exotic molecules such as positronium hydride have also been made. Bonds Bonds between hydrogen and the other elements range from highly to somewhat covalent. Some hydrides, e.g. boron hydrides, do not conform to classical electron-counting rules and the bonding is described in terms of multi-centered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Subrahmanyan Chandrasekhar
Subrahmanyan Chandrasekhar (; ) (19 October 1910 – 21 August 1995) was an Indian-American theoretical physicist who spent his professional life in the United States. He shared the 1983 Nobel Prize for Physics with William A. Fowler for "...theoretical studies of the physical processes of importance to the structure and evolution of the stars". His mathematical treatment of stellar evolution yielded many of the current theoretical models of the later evolutionary stages of massive stars and black holes. Many concepts, institutions, and inventions, including the Chandrasekhar limit and the Chandra X-Ray Observatory, are named after him. Chandrasekhar worked on a wide variety of problems in physics during his lifetime, contributing to the contemporary understanding of stellar structure, white dwarfs, stellar dynamics, stochastic process, radiative transfer, the quantum theory of the hydrogen anion, hydrodynamic and hydromagnetic stability, turbulence, equilibrium and the stabi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spicule (solar Physics)
In solar physics, a spicule, also known as a fibril or mottle, is a dynamic jet of plasma in the Sun's chromosphere about 300 km in diameter.Quantifying Spicules, Tiago M. D. Pereira, Bart De Pontieu, and Mats Carlsson, ''The Astrophysical Journal'' 759, #1 (October 2012), pp. 18-34, , . They move upwards with speeds between 15 and 110 km/s from the photosphere and last a few minutes each. They were discovered in 1877 by Angelo Secchi, but the physical mechanism that generates them is still hotly debated. Description Spicules last for about 15 minutes; at the solar limb they appear elongated (if seen on the disk, they are known as "mottles" or "fibrils"). They are usually associated with regions of high magnetic flux; their mass flux is about 100 times that of the solar wind. They rise at a rate of 20 km/s (or 72,000 km/h) and can reach several thousand kilometers in height before collapsing and fading away. Prevalence There are about 3,000,000 active spicul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)


