|
SECC (metal)
Electrogalvanizing is a process in which a layer of zinc is bonded to steel in order to protect against corrosion. The process involves electroplating, running a current of electricity through a saline/zinc solution with a zinc anode and steel conductor. Such Zinc electroplating or Zinc alloy electroplating maintains a dominant position among other electroplating process options, based upon electroplated tonnage per annum. According to the International Zinc Association, more than 5 million tons are used yearly for both hot dip galvanizing and electroplating. The plating of zinc was developed at the beginning of the 20th century. At that time, the electrolyte was cyanide based. A significant innovation occurred in the 1960s, with the introduction of the first acid chloride based electrolyte. The 1980s saw a return to alkaline electrolytes, only this time, without the use of cyanide. The most commonly used electrogalvanized cold rolled steel is SECC, acronym of "Steel, Electrogalvaniz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size.The elements are from different metal groups. See periodic table. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity ( electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
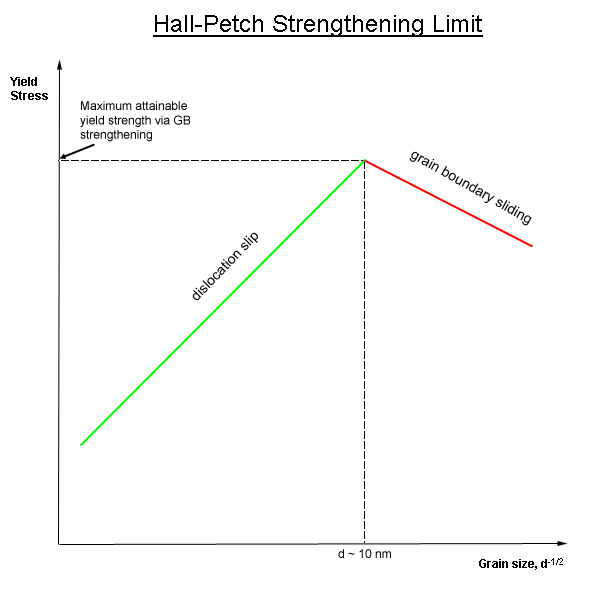
Grain Boundary Strengthening
In materials science, grain-boundary strengthening (or Hall–Petch strengthening) is a method of strengthening materials by changing their average crystallite (grain) size. It is based on the observation that grain boundaries are insurmountable borders for dislocations and that the number of dislocations within a grain has an effect on how stress builds up in the adjacent grain, which will eventually activate dislocation sources and thus enabling deformation in the neighbouring grain as well. So, by changing grain size, one can influence the number of dislocations piled up at the grain boundary and yield strength. For example, heat treatment after plastic deformation and changing the rate of solidification are ways to alter grain size.W.D. Callister. Fundamentals of Materials Science and Engineering, 2nd ed. Wiley & Sons. pp. 252. Theory In grain-boundary strengthening, the grain boundaries act as pinning points impeding further dislocation propagation. Since the lattice struct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, produced by reductive smelting, is a hard, lustrous, silver-gray metal. Cobalt-based blue pigments ( cobalt blue) have been used since ancient times for jewelry and paints, and to impart a distinctive blue tint to glass, but the color was for a long time thought to be due to the known metal bismuth. Miners had long used the name ''kobold ore'' (German for ''goblin ore'') for some of the blue-pigment-producing minerals; they were so named because they were poor in known metals, and gave poisonous arsenic-containing fumes when smelted. In 1735, such ores were found to be reducible to a new metal (the first discovered since ancient times), and this was ultimately named for the ''kobold''. Today, some cobalt is produced specifically from one of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in front of oxygen (32.1% and 30.1%, respectively), forming much of Earth's outer and inner core. It is the fourth most common element in the Earth's crust. In its metallic state, iron is rare in the Earth's crust, limited mainly to deposition by meteorites. Iron ores, by contrast, are among the most abundant in the Earth's crust, although extracting usable metal from them requires kilns or furnaces capable of reaching or higher, about higher than that required to smelt copper. Humans started to master that process in Eurasia during the 2nd millennium BCE and the use of iron tools and weapons began to displace copper alloys, in some regions, only around 1200 BCE. That event is considered the transition from the Bronze Age to the Iron A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size.The elements are from different metal groups. See periodic table. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity ( electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coefficient Of Friction
Friction is the force resisting the relative motion of solid surfaces, fluid layers, and material elements sliding against each other. There are several types of friction: *Dry friction is a force that opposes the relative lateral motion of two solid surfaces in contact. Dry friction is subdivided into ''static friction'' ("stiction") between non-moving surfaces, and ''kinetic friction'' between moving surfaces. With the exception of atomic or molecular friction, dry friction generally arises from the interaction of surface features, known as asperities (see Figure 1). *Fluid friction describes the friction between layers of a viscous fluid that are moving relative to each other. *Lubricated friction is a case of fluid friction where a lubricant fluid separates two solid surfaces. *Skin friction is a component of drag, the force resisting the motion of a fluid across the surface of a body. *Internal friction is the force resisting motion between the elements making up a s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wear Resistance
Wear is the damaging, gradual removal or deformation of material at solid surfaces. Causes of wear can be mechanical (e.g., erosion) or chemical (e.g., corrosion). The study of wear and related processes is referred to as tribology. Wear in machine elements, together with other processes such as fatigue and creep, causes functional surfaces to degrade, eventually leading to material failure or loss of functionality. Thus, wear has large economic relevance as first outlined in the Jost Report. Abrasive wear alone has been estimated to cost 1-4% of the gross national product of industrialized nations. Wear of metals occurs by plastic displacement of surface and near-surface material and by detachment of particles that form wear debris. The particle size may vary from millimeters to nanometers. This process may occur by contact with other metals, nonmetallic solids, flowing liquids, solid particles or liquid droplets entrained in flowing gasses. The wear rate is affected by fa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tool Steel
Tool steel is any of various carbon steels and alloy steels that are particularly well-suited to be made into tools and tooling, including cutting tools, dies, hand tools, knives, and others. Their suitability comes from their distinctive hardness, resistance to abrasion and deformation, and their ability to hold a cutting edge at elevated temperatures. As a result, tool steels are suited for use in the shaping of other materials, as for example in cutting, machining, stamping, or forging. With a carbon content between 0.5% and 1.5%, tool steels are manufactured under carefully controlled conditions to produce the required quality. The presence of carbides in their matrix plays the dominant role in the qualities of tool steel. The four major alloying elements that form carbides in tool steel are: tungsten, chromium, vanadium and molybdenum. The rate of dissolution of the different carbides into the austenite form of the iron determines the high-temperature performance of st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the most abundant chemical substance in the universe, constituting roughly 75% of all normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of protons, the nuclei of hydrogen, occurred during the first second after the Big Bang. The emergence of neutral hydrogen atoms throughout the universe occurred about 370,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Embrittlement
Hydrogen embrittlement (HE), also known as hydrogen-assisted cracking or hydrogen-induced cracking (HIC), is a reduction in the ductility of a metal due to absorbed hydrogen. Hydrogen atoms are small and can permeate solid metals. Once absorbed, hydrogen lowers the stress required for cracks in the metal to initiate and propagate, resulting in embrittlement. Hydrogen embrittlement occurs most notably in steels, as well as in iron, nickel, titanium, cobalt, and their alloys. Copper, aluminium, and stainless steels are less susceptible to hydrogen embrittlement. The essential facts about the nature of hydrogen embrittlement have been known since the 19th century. Hydrogen embrittlement is maximised at around room temperature in steels, and most metals are relatively immune to hydrogen embrittlement at temperatures above 150 °C. Hydrogen embrittlement requires the presence of both atomic ("diffusible") hydrogen and a mechanical stress to induce crack growth, although that str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistant typically need an additional 11% chromium. Because of its high tensile strength and low cost, steel is used in buildings, infrastructure, tools, ships, trains, cars, machines, electrical appliances, weapons, and rockets. Iron is the base metal of steel. Depending on the temperature, it can take two crystalline forms (allotropic forms): body-centred cubic and face-centred cubic. The interaction of the allotropes of iron with the alloying elements, primarily carbon, gives steel and cast iron their range of unique properties. In pure iron, the crystal structure has relatively little resistance to the iron atoms slipping past one another, and so pure iron is quite ductile, or soft and easily formed. In steel, small amounts of carbon, other ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrotrope
A hydrotrope is a compound that solubilizes hydrophobic compounds in aqueous solutions by means other than micellar solubilization. Typically, hydrotropes consist of a hydrophilic part and a hydrophobic part (similar to surfactants), but the hydrophobic part is generally too small to cause spontaneous self-aggregation. Hydrotropes do not have a critical concentration above which self-aggregation spontaneously starts to occur (as found for micelle- and vesicle-forming surfactants, which have a critical micelle concentration (cmc) and a critical vesicle concentration (cvc)). Instead, some hydrotropes aggregate in a step-wise self-aggregation process, gradually increasing aggregation size. However, many hydrotropes do not seem to self-aggregate at all, unless a solubilizate has been added. Examples of hydrotropes include urea, tosylate, cumenesulfonate and xylenesulfonate. The term ''hydrotropy'' was originally put forward by Carl Neuberg to describe the increase in the solubility o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |