|
S-type Star
An S-type star (or just S star) is a cool giant with approximately equal quantities of carbon and oxygen in its atmosphere. The class was originally defined in 1922 by Paul Merrill for stars with unusual absorption lines and molecular bands now known to be due to s-process elements. The bands of zirconium monoxide (ZrO) are a defining feature of the S stars. The carbon stars have more carbon than oxygen in their atmospheres. In most stars, such as class M giants, the atmosphere is richer in oxygen than carbon and they are referred to as ''oxygen-rich stars''. S-type stars are intermediate between carbon stars and normal giants. They can be grouped into two classes: ''intrinsic'' S stars, which owe their spectra to convection of fusion products and s-process elements to the surface; and ''extrinsic'' S stars, which are formed through mass transfer in a binary system. The intrinsic S-type stars are on the most luminous portion of the asymptotic giant branch, a stage of their ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
W Aquilae Binary
W, or w, is the twenty-third and fourth-to-last letter of the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. It represents a consonant, but in some languages it represents a vowel. Its name in English is ''double-u'',Pronounced in formal situations, but colloquially often , , or , with a silent ''l''. plural ''double-ues''. History The classical Latin alphabet, from which the modern European alphabets derived, did not have the "W' character. The "W" sounds were represented by the Latin letter " V" (at the time, not yet distinct from " U"). The sounds (spelled ) and (spelled ) of Classical Latin developed into a bilabial fricative between vowels in Early Medieval Latin. Therefore, no longer adequately represented the labial-velar approximant sound of Germanic phonology. The Germanic phoneme was therefore written as or ( and becoming distinct only by the Early Modern period) by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Slow Irregular Variable
A slow irregular variable (ascribed the GCVS types L, LB and LC) is a variable star that exhibit no or very poorly defined periodicity in their slowly changing light emissions. These stars have often been little-studied, and once more is learnt about them, they are reclassified into other categories such as semiregular variables. Nomenclature Irregular variable stars were first given acronyms based on the letter "I": ''Ia'', ''Ib''. and ''Ic''. These were later refined so that the I codes were used "nebular" or "rapidly irregular" variable stars such as T Tauri and Orion variables. The remaining irregular stars, cool slowly varying giants and supergiants of type Ib or Ic were reassigned to Lb and Lc. When the General Catalogue of Variable Stars standardised its acronyms to be all uppercase, the codes LB and LC were used. Type Lb ''Slow irregular variables of late spectral types ( K, M, C, S); as a rule, they are giants'' The GCVS also claims to give this type to slow irregula ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mira Variable
Mira variables (named for the prototype star Mira) are a class of pulsating stars characterized by very red colours, pulsation periods longer than 100 days, and amplitudes greater than one magnitude in infrared and 2.5 magnitude at visual wavelengths. They are red giants in the very late stages of stellar evolution, on the asymptotic giant branch (AGB), that will expel their outer envelopes as planetary nebulae and become white dwarfs within a few million years. Mira variables are stars massive enough that they have undergone helium fusion in their cores but are less than two solar masses, stars that have already lost about half their initial mass. However, they can be thousands of times more luminous than the Sun due to their very large distended envelopes. They are pulsating due to the entire star expanding and contracting. This produces a change in temperature along with radius, both of which factors cause the variation in luminosity. The pulsation depends on the mass a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
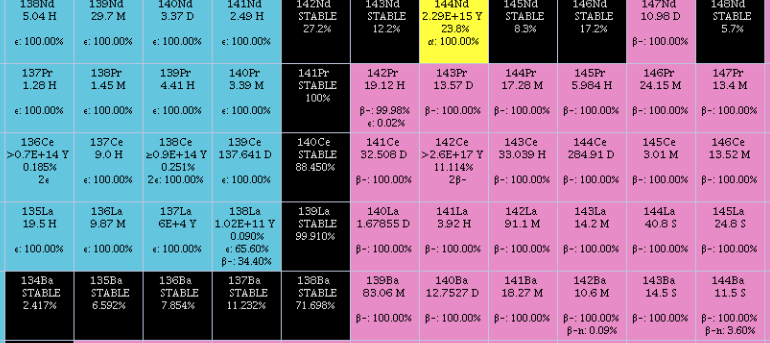
Technetium Star
A technetium star, or more properly a Tc-rich star, is a star whose stellar spectrum contains absorption lines of the light radioactive metal technetium. The most stable isotope of technetium is 97Tc with a half-life of 4.21 million years, which is too short a time to allow the metal to be material from before the star's formation. Therefore, the detection in 1952 of technetium in stellar spectra provided unambiguous proof of nucleosynthesis in stars, one of the more extreme cases being R Geminorum. Stars containing technetium belong to the class of asymptotic giant branch stars (AGB)—stars that are like red giants, but with a slightly higher luminosity, and which burn hydrogen in an inner shell. Members of this class of stars switch to helium shell burning with an interval of some 100,000 years, in "dredge-ups". Technetium stars belong to the classes M, MS, S, SC and C-N. They are most often variable stars of the long period variable types. Current research indica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Technetium
Technetium is a chemical element with the symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. All available technetium is produced as a synthetic element. Naturally occurring technetium is a spontaneous fission product in uranium ore and thorium ore, the most common source, or the product of neutron capture in molybdenum ores. This silvery gray, crystalline transition metal lies between manganese and rhenium in group 7 of the periodic table, and its chemical properties are intermediate between those of both adjacent elements. The most common naturally occurring isotope is 99Tc, in traces only. Many of technetium's properties had been predicted by Dmitri Mendeleev before it was discovered. Mendeleev noted a gap in his periodic table and gave the undiscovered element the provisional name '' ekamanganese'' (''Em''). In 1937, technetium (specifically the technetium-97 isotope) became the first predominantly artificial element to be produced ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Period 5 Element
The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour begins to repeat, meaning that elements with similar behaviour fall into the same vertical columns. The fifth period contains 18 elements, beginning with rubidium and ending with xenon. As a rule, period 5 elements fill their 5s shells first, then their 4d, and 5p shells, in that order; however, there are exceptions, such as rhodium. Physical properties This period contains technetium, one of the two elements until lead that has no stable isotopes (along with promethium), as well as molybdenum and iodine, two of the heaviest elements with a known biological role, and Niobium has the largest magnetic known penetration depth of all the elements. Zirconium is one of the main components of zircon crystals, currently the oldest known minerals in the earth's crust. Many later transition me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( passivation) somewhat stabilizes the free metal against further oxidation. Spanish scientist Andrés Manuel del Río discovered compounds of vanadium in 1801 in Mexico by analyzing a new lead-bearing mineral he called "brown lead". Though he initially presumed its qualities were due to the presence of a new element, he was later erroneously convinced by French chemist Hippolyte Victor Collet-Descotils that the element was just chromium. Then in 1830, Nils Gabriel Sefström generated chlorides of vanadium, thus proving there was a new element, and named it "vanadium" after the Scandinavian goddess of beauty and fertility, Vanadís (Freyja). The name was based on the wide range of colors found in vanadium compounds. Del Rio's lead mineral w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between lanthanum and lutetium in the periodic table, of which lanthanum is the first and the prototype. Lanthanum is traditionally counted among the rare earth elements. Like most other rare earth elements, the usual oxidation state is +3. Lanthanum has no biological role in humans but is essential to some bacteria. It is not particularly toxic to humans but does show some antimicrobial activity. Lanthanum usually occurs together with cerium and the other rare earth elements. Lanthanum was first found by the Swedish chemist Carl Gustaf Mosander in 1839 as an impurity in cerium nitrate – hence the name ''lanthanum'', from the Ancient Greek (), meaning 'to lie hidden'. Although it is classified as a rare earth element, lanthanum is the 28th most ab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Barium
Barium is a chemical element with the symbol Ba and atomic number 56. It is the fifth element in group 2 and is a soft, silvery alkaline earth metal. Because of its high chemical reactivity, barium is never found in nature as a free element. The most common minerals of barium are baryte (barium sulfate, BaSO4) and witherite ( barium carbonate, BaCO3). The name ''barium'' originates from the alchemical derivative "baryta", from Greek (), meaning 'heavy'. ''Baric'' is the adjectival form of barium. Barium was identified as a new element in 1774, but not reduced to a metal until 1808 with the advent of electrolysis. Barium has few industrial applications. Historically, it was used as a getter for vacuum tubes and in oxide form as the emissive coating on indirectly heated cathodes. It is a component of YBCO ( high-temperature superconductors) and electroceramics, and is added to steel and cast iron to reduce the size of carbon grains within the microstructure. Barium compou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to air. Strontium has physical and chemical properties similar to those of its two vertical neighbors in the periodic table, calcium and barium. It occurs naturally mainly in the minerals celestine and strontianite, and is mostly mined from these. Both strontium and strontianite are named after Strontian, a village in Scotland near which the mineral was discovered in 1790 by Adair Crawford and William Cruickshank; it was identified as a new element the next year from its crimson-red flame test color. Strontium was first isolated as a metal in 1808 by Humphry Davy using the then newly discovered process of electrolysis. During the 19th century, strontium was mostly used in the production of sugar from sugar beets (see str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yttrium
Yttrium is a chemical element with the symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often been classified as a " rare-earth element". Yttrium is almost always found in combination with lanthanide elements in rare-earth minerals, and is never found in nature as a free element. 89Y is the only stable isotope, and the only isotope found in the Earth's crust. The most important uses of yttrium are LEDs and phosphors, particularly the red phosphors in television set cathode ray tube displays. Yttrium is also used in the production of electrodes, electrolytes, electronic filters, lasers, superconductors, various medical applications, and tracing various materials to enhance their properties. Yttrium has no known biological role. Exposure to yttrium compounds can cause lung disease in humans. The element is named after ''ytterbite'', a mineral first identified in 1787 by the chemist Carl Axel Arrheni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide ( lye) is used in soap manufacture, and sodium chloride ( edible salt) is a de-icing agent and a nutrient for animals i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |