Yttrium on:
[Wikipedia]
[Google]
[Amazon]
Yttrium is a
 As a trivalent transition metal, yttrium forms various inorganic compounds, generally in the oxidation state of +3, by giving up all three of its valence electrons. A good example is
As a trivalent transition metal, yttrium forms various inorganic compounds, generally in the oxidation state of +3, by giving up all three of its valence electrons. A good example is
 Yttrium isotopes are among the most common products of the
Yttrium isotopes are among the most common products of the
 Johan Gadolin at the University of Åbo identified a new oxide (or "
Johan Gadolin at the University of Åbo identified a new oxide (or "

 Rare-earth elements (REEs) come mainly from four sources:
* Carbonate and fluoride containing ores such as the LREE bastnäsite (
Rare-earth elements (REEs) come mainly from four sources:
* Carbonate and fluoride containing ores such as the LREE bastnäsite ( /nowiki>(Ce, La, etc.)(CO3)F Ce,_lanthanum.html"_;"title="cerium.html"_;"title="/nowiki>(cerium">Ce,_lanthanum">La,_etc.) Ce,_lanthanum.html"_;"title="cerium.html"_;"title="/nowiki>(cerium">Ce,_lanthanum">La,_etc.)phosphate">PO4.html" ;"title="phosphate.html" ;"title="cerium">Ce,_lanthanum.html" ;"title="cerium.html" ;"title="/nowiki>(cerium">Ce, lanthanum">La, etc.)phosphate">PO4">phosphate.html" ;"title="cerium">Ce,_lanthanum.html" ;"title="cerium.html" ;"title="/nowiki>(cerium">Ce, lanthanum">La, etc.)phosphate">PO4/nowiki>), which is mostly phosphate, is a placer deposit of sand created by the transportation and gravitational separation of eroded granite. Monazite as a LREE ore contains 2% (or 3%) Stwertka 1998, p. 116 yttrium. The largest deposits were found in India and Brazil in the early 20th century, making those two countries the largest producers of yttrium in the first half of that century. Of the monazite group, the Ce-dominant member, monazite-(Ce), is the most common one.
*
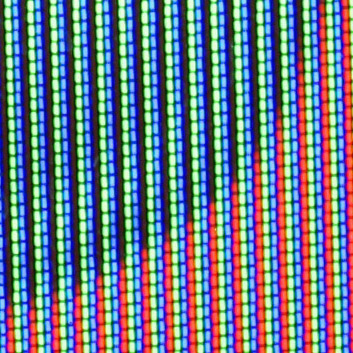 The red component of
The red component of
 Yttrium is used in the production of a large variety of synthetic garnets, and yttria is used to make yttrium iron garnets (, also "YIG"), which are very effective
Yttrium is used in the production of a large variety of synthetic garnets, and yttria is used to make yttrium iron garnets (, also "YIG"), which are very effective
 Yttrium is a key ingredient in the yttrium barium copper oxide (YBa2Cu3O7, aka 'YBCO' or '1-2-3') superconductor developed at the
Yttrium is a key ingredient in the yttrium barium copper oxide (YBa2Cu3O7, aka 'YBCO' or '1-2-3') superconductor developed at the
LYP cells
have essentially same
Yttrium by Paul C.W. Chu at acs.org
at '' The Periodic Table of Videos'' (University of Nottingham) *
Encyclopedia of Geochemistry - Yttrium
{{Authority control Chemical elements Transition metals Deoxidizers Chemical elements with hexagonal close-packed structure
chemical element
A chemical element is a species of atoms that have a given number of protons in their atomic nucleus, nuclei, including the pure Chemical substance, substance consisting only of that species. Unlike chemical compounds, chemical elements canno ...
with the symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Y and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
39. It is a silvery-metallic transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
chemically similar to the lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yt ...
s and has often been classified as a " rare-earth element". Yttrium is almost always found in combination with lanthanide elements in rare-earth mineral
A rare-earth mineral contains one or more rare-earth elements as major metal constituents. Rare-earth minerals are usually found in association with alkaline to peralkaline igneous complexes, in pegmatites associated with alkaline magmas and ...
s, and is never found in nature as a free element. 89Y is the only stable isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
, and the only isotope found in the Earth's crust
Earth's crust is Earth's thin outer shell of rock, referring to less than 1% of Earth's radius and volume. It is the top component of the lithosphere, a division of Earth's layers that includes the crust and the upper part of the mantle. The ...
.
The most important uses of yttrium are LEDs and phosphor
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or v ...
s, particularly the red phosphors in television set cathode ray tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms ( oscilloscope), ...
displays. Yttrium is also used in the production of electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or air). Electrodes are essential parts of batteries that can consist of a variety of materials ...
s, electrolyte
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon ...
s, electronic filters
Electronic filters are a type of signal processing filter in the form of electrical circuits. This article covers those filters consisting of lumped electronic components, as opposed to distributed-element filters. That is, using component ...
, laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word "laser" is an acronym for "light amplification by stimulated emission of radiation". The ...
s, superconductors, various medical applications, and tracing various materials to enhance their properties.
Yttrium has no known biological
Biology is the scientific study of life. It is a natural science with a broad scope but has several unifying themes that tie it together as a single, coherent field. For instance, all organisms are made up of cells that process hereditary in ...
role. Exposure to yttrium compounds can cause lung disease
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either side ...
in humans.
The element is named after ''ytterbite
Gadolinite, sometimes known as ytterbite, is a silicate mineral consisting principally of the silicates of cerium, lanthanum, neodymium, yttrium, beryllium, and iron with the formula . It is called gadolinite-(Ce) or gadolinite-(Y), depending on ...
'', a mineral first identified in 1787 by the chemist Carl Axel Arrhenius. He named the mineral after the village of Ytterby
Ytterby () is a village on the Swedish island of Resarö, in Vaxholm Municipality in the Stockholm archipelago. Today the residential area is dominated by suburban homes.
The name of the village translates to "outer village". Ytterby is per ...
, in Sweden
Sweden, formally the Kingdom of Sweden,The United Nations Group of Experts on Geographical Names states that the country's formal name is the Kingdom of SwedenUNGEGN World Geographical Names, Sweden./ref> is a Nordic countries, Nordic c ...
, where it had been discovered. When one of the chemicals in ytterbite was later found to be the previously unidentified element, yttrium, the element was then named after the mineral.
Characteristics
Properties
Yttrium is a soft, silver-metallic, lustrous and highly crystallinetransition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
in group 3. As expected by periodic trends, it is less electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the ...
than its predecessor in the group, scandium
Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the Lanthanides. It was discovered in ...
, and less electronegative than the next member of period 5
The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour begins to repeat, meaning that elements with si ...
, zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'' ...
; additionally, it is more electronegative than lanthanum
Lanthanum is a chemical element with the symbol La and atomic number 57. It is a soft, ductile, silvery-white metal that tarnishes slowly when exposed to air. It is the eponym of the lanthanide series, a group of 15 similar elements between l ...
, but less electronegative than lutetium due to the lanthanide contraction. Yttrium is the first d-block element in the fifth period.
The pure element is relatively stable in air in bulk form, due to passivation of a protective oxide () film that forms on the surface. This film can reach a thickness of 10 µm
The micrometre ( international spelling as used by the International Bureau of Weights and Measures; SI symbol: μm) or micrometer ( American spelling), also commonly known as a micron, is a unit of length in the International System of Uni ...
when yttrium is heated to 750 ° C in water vapor
(99.9839 °C)
, -
, Boiling point
,
, -
, specific gas constant
, 461.5 J/( kg·K)
, -
, Heat of vaporization
, 2.27 MJ/kg
, -
, Heat capacity
, 1.864 kJ/(kg·K)
Water vapor, water vapour or aqueous vapor is the gaseous p ...
. When finely divided, however, yttrium is very unstable in air; shavings or turnings of the metal can ignite in air at temperatures exceeding 400 °C. Yttrium nitride (YN) is formed when the metal is heated to 1000 °C in nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at se ...
.
Similarity to the lanthanides
The similarities of yttrium to thelanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yt ...
s are so strong that the element has historically been grouped with them as a rare-earth element, and is always found in nature together with them in rare-earth mineral
A rare-earth mineral contains one or more rare-earth elements as major metal constituents. Rare-earth minerals are usually found in association with alkaline to peralkaline igneous complexes, in pegmatites associated with alkaline magmas and ...
s. Emsley 2001, p. 498 Chemically, yttrium resembles those elements more closely than its neighbor in the periodic table, scandium
Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the Lanthanides. It was discovered in ...
, Daane 1968, p. 810. and if physical properties were plotted against atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
, it would have an apparent number of 64.5 to 67.5, placing it between the lanthanides gadolinium and erbium
Erbium is a chemical element with the symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare-earth element, ...
. Daane 1968, p. 815.
It often also falls in the same range for reaction order, resembling terbium and dysprosium
Dysprosium is the chemical element with the symbol Dy and atomic number 66. It is a rare-earth element in the lanthanide series with a metallic silver luster. Dysprosium is never found in nature as a free element, though, like other lanthanide ...
in its chemical reactivity. Yttrium is so close in size to the so-called 'yttrium group' of heavy lanthanide ions that in solution, it behaves as if it were one of them. Even though the lanthanides are one row farther down the periodic table than yttrium, the similarity in atomic radius may be attributed to the lanthanide contraction.
One of the few notable differences between the chemistry of yttrium and that of the lanthanides is that yttrium is almost exclusively trivalent, whereas about half the lanthanides can have valences other than three; nevertheless, only for four of the fifteen lanthanides are these other valences important in aqueous solution ( CeIV, SmII, EuII, and YbII). Daane 1968, p. 817
Compounds and reactions
 As a trivalent transition metal, yttrium forms various inorganic compounds, generally in the oxidation state of +3, by giving up all three of its valence electrons. A good example is
As a trivalent transition metal, yttrium forms various inorganic compounds, generally in the oxidation state of +3, by giving up all three of its valence electrons. A good example is yttrium(III) oxide
Yttrium oxide, also known as yttria, is Y2 O3. It is an air-stable, white solid substance.
The thermal conductivity of yttrium oxide is 27 W/(m·K).
Uses Phosphors
Yttria is widely used to make Eu:YVO4 and Eu:Y2O3 phosphors that give the red ...
(), also known as yttria, a six- coordinate white solid.
Yttrium forms a water-insoluble fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts ty ...
, hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. ...
, and oxalate, but its bromide, chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride s ...
, iodide, nitrate
Nitrate is a polyatomic ion with the chemical formula . Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are soluble in water. An example of an insolu ...
and sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
are all soluble in water. The Y3+ ion is colorless in solution because of the absence of electrons in the d and f electron shells.
Water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
readily reacts with yttrium and its compounds to form . Concentrated nitric and hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colourless, acidic and highly corrosive. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepres ...
s do not rapidly attack yttrium, but other strong acids do.
With halogen
The halogens () are a group in the periodic table consisting of five or six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts). In the modern IUPAC nomenclature, this grou ...
s, yttrium forms trihalides such as yttrium(III) fluoride
Yttrium(III) fluoride is an inorganic chemical compound with the chemical formula Y F3. It is not known naturally in 'pure' form. The fluoride minerals containing essential yttrium include tveitite-(Y) (Y,Na)6Ca6Ca6F42 and gagarinite-(Y) NaCaY( ...
(), yttrium(III) chloride (), and yttrium(III) bromide
Yttrium(III) bromide is an inorganic compound with the chemical formula YBr3. It is a white solid. Anhydrous yttrium(III) bromide can be produced by reacting yttrium oxide or yttrium(III) bromide hydrate and ammonium bromide. The reaction proceeds ...
() at temperatures above roughly 200 °C. Similarly, carbon
Carbon () is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent—its atom making four electrons available to form covalent chemical bonds. It belongs to group 14 of the periodic table. Carbon ma ...
, phosphorus
Phosphorus is a chemical element with the symbol P and atomic number 15. Elemental phosphorus exists in two major forms, white phosphorus and red phosphorus, but because it is highly reactive, phosphorus is never found as a free element on Ea ...
, selenium
Selenium is a chemical element with the symbol Se and atomic number 34. It is a nonmetal (more rarely considered a metalloid) with properties that are intermediate between the elements above and below in the periodic table, sulfur and tellurium, ...
, silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ...
and sulfur
Sulfur (or sulphur in British English) is a chemical element with the symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formul ...
all form binary compounds with yttrium at elevated temperatures.
Organoyttrium chemistry
Organoyttrium chemistry is the study of compounds containing carbon-yttrium
Yttrium is a chemical element with the symbol Y and atomic number 39. It is a silvery-metallic transition metal chemically similar to the lanthanides and has often ...
is the study of compounds containing carbon–yttrium bonds. A few of these are known to have yttrium in the oxidation state 0. (The +2 state has been observed in chloride melts, and +1 in oxide clusters in the gas phase.) Some trimerization reactions were generated with organoyttrium compounds as catalysts. These syntheses use as a starting material, obtained from and concentrated hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dige ...
and ammonium chloride.
Hapticity is a term to describe the coordination of a group of contiguous atoms of a ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's elect ...
bound to the central atom; it is indicated by the Greek character ''eta'', η. Yttrium complexes were the first examples of complexes where carboranyl ligands were bound to a d0-metal center through a η7-hapticity. Vaporization of the graphite intercalation compound
Graphite intercalation compounds are complex materials having a formula where the ion or is inserted ( intercalated) between the oppositely charged carbon layers. Typically ''m'' is much less than 1. These materials are deeply colored solids t ...
s graphite–Y or graphite– leads to the formation of endohedral fullerene
Endohedral fullerenes, also called endofullerenes, are fullerenes that have additional atoms, ions, or clusters enclosed within their inner spheres. The first lanthanum C60 complex called La@C60 was synthesized in 1985. The @ (at sign) in the ...
s such as Y@C82. Electron spin resonance studies indicated the formation of Y3+ and (C82)3− ion pairs. The carbide
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.
Interstitial / Metallic carbides
The carbides of t ...
s Y3C, Y2C, and YC2 can be hydrolyzed to form hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or ...
s.
Isotopes and nucleosynthesis
Yttrium in theSolar System
The Solar System Capitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Solar ...
was created through stellar nucleosynthesis
Stellar nucleosynthesis is the creation (nucleosynthesis) of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. A ...
, mostly by the s-process
The slow neutron-capture process, or ''s''-process, is a series of reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The ''s''-process is responsible for the creation ( nucleosynthesis) of approxim ...
(≈72%), but also by the r-process
In nuclear astrophysics, the rapid neutron-capture process, also known as the ''r''-process, is a set of nuclear reactions that is responsible for the creation of approximately half of the atomic nuclei heavier than iron, the "heavy elements", ...
(≈28%). The r-process consists of rapid neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons ...
by lighter elements during supernova
A supernova is a powerful and luminous explosion of a star. It has the plural form supernovae or supernovas, and is abbreviated SN or SNe. This transient astronomical event occurs during the last evolutionary stages of a massive star or whe ...
explosions. The s-process is a slow neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
capture of lighter elements inside pulsating red giant
A red giant is a luminous giant star of low or intermediate mass (roughly 0.3–8 solar masses ()) in a late phase of stellar evolution. The outer atmosphere is inflated and tenuous, making the radius large and the surface temperature around o ...
stars.
 Yttrium isotopes are among the most common products of the
Yttrium isotopes are among the most common products of the nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
of uranium in nuclear explosions and nuclear reactors. In the context of nuclear waste management, the most important isotopes of yttrium are 91Y and 90Y, with half-lives of 58.51 days and 64 hours, respectively. Though 90Y has a short half-life, it exists in secular equilibrium with its long-lived parent isotope, strontium-90 (90Sr) with a half-life of 29 years.
All group 3 elements have an odd atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
, and therefore few stable isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
s. Scandium
Scandium is a chemical element with the symbol Sc and atomic number 21. It is a silvery-white metallic d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the Lanthanides. It was discovered in ...
has one stable isotope, and yttrium itself has only one stable isotope, 89Y, which is also the only isotope that occurs naturally. However, the lanthanide rare earths contain elements of even atomic number and many stable isotopes. Yttrium-89 is thought to be more abundant than it otherwise would be, due in part to the s-process, which allows enough time for isotopes created by other processes to decay by electron emission
In physics, electron emission is the ejection of an electron from the surface of matter, or, in beta decay (β− decay), where a beta particle (a fast energetic electron or positron) is emitted from an atomic nucleus transforming the original nucl ...
(neutron → proton). Such a slow process tends to favor isotopes with atomic mass number
The mass number (symbol ''A'', from the German word ''Atomgewicht'' tomic weight, also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approxi ...
s (A = protons + neutrons) around 90, 138 and 208, which have unusually stable atomic nuclei
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron ...
with 50, 82, and 126 neutrons, respectively. This stability is thought to result from their very low neutron-capture cross-section. . Electron emission of isotopes with those mass numbers is simply less prevalent due to this stability, resulting in them having a higher abundance. 89Y has a mass number close to 90 and has 50 neutrons in its nucleus.
At least 32 synthetic isotopes of yttrium have been observed, and these range in atomic mass number
The mass number (symbol ''A'', from the German word ''Atomgewicht'' tomic weight, also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approxi ...
from 76 to 108. The least stable of these is 106Y with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
of >150 ns (76Y has a half-life of >200 ns) and the most stable is 88Y with a half-life of 106.626 days. Apart from the isotopes 91Y, 87Y, and 90Y, with half-lives of 58.51 days, 79.8 hours, and 64 hours, respectively, all the other isotopes have half-lives of less than a day and most of less than an hour.
Yttrium isotopes with mass numbers at or below 88 decay primarily by positron emission (proton → neutron) to form strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is e ...
( Z = 38) isotopes. Yttrium isotopes with mass numbers at or above 90 decay primarily by electron emission (neutron → proton) to form zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'' ...
(Z = 40) isotopes. Isotopes with mass numbers at or above 97 are also known to have minor decay paths of β− delayed neutron emission.
Yttrium has at least 20 metastable ("excited") isomers ranging in mass number from 78 to 102. Multiple excitation states have been observed for 80Y and 97Y. While most of yttrium's isomers are expected to be less stable than their ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
, 78mY, 84mY, 85mY, 96mY, 98m1Y, 100mY, and 102mY have longer half-lives than their ground states, as these isomers decay by beta decay rather than isomeric transition
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy higher energy levels than in the ground state of the same nucleus. "Metastable" describes nuclei whose excited states have ...
.
History
In 1787, part-time chemist Carl Axel Arrhenius found a heavy black rock in an old quarry near the Swedish village ofYtterby
Ytterby () is a village on the Swedish island of Resarö, in Vaxholm Municipality in the Stockholm archipelago. Today the residential area is dominated by suburban homes.
The name of the village translates to "outer village". Ytterby is per ...
(now part of the Stockholm Archipelago). Thinking it was an unknown mineral containing the newly discovered element tungsten
Tungsten, or wolfram, is a chemical element with the symbol W and atomic number 74. Tungsten is a rare metal found naturally on Earth almost exclusively as compounds with other elements. It was identified as a new element in 1781 and first isol ...
, Emsley 2001, p. 496 he named it ''ytterbite'' and sent samples to various chemists for analysis. Van der Krogt 2005
 Johan Gadolin at the University of Åbo identified a new oxide (or "
Johan Gadolin at the University of Åbo identified a new oxide (or "earth
Earth is the third planet from the Sun and the only astronomical object known to harbor life. While large volumes of water can be found throughout the Solar System, only Earth sustains liquid surface water. About 71% of Earth's sur ...
") in Arrhenius' sample in 1789, and published his completed analysis in 1794. Anders Gustaf Ekeberg
Anders Gustaf Ekeberg (Stockholm, Sweden, 16 January 1767 – Uppsala, Sweden, 11 February 1813) was a Swedish analytical chemist who discovered tantalum in 1802. - subscription required
He was notably deaf.
Education
Anders Gustav Ekeberg w ...
confirmed the identification in 1797 and named the new oxide ''yttria''. In the decades after Antoine Lavoisier developed the first modern definition of chemical element
A chemical element is a species of atoms that have a given number of protons in their atomic nucleus, nuclei, including the pure Chemical substance, substance consisting only of that species. Unlike chemical compounds, chemical elements canno ...
s, it was believed that earths could be reduced to their elements, meaning that the discovery of a new earth was equivalent to the discovery of the element within, which in this case would have been ''yttrium''.
Friedrich Wöhler
Friedrich Wöhler () FRS(For) Hon FRSE (31 July 180023 September 1882) was a German chemist known for his work in inorganic chemistry, being the first to isolate the chemical elements beryllium and yttrium in pure metallic form. He was the fi ...
is credited with first isolating the metal in 1828 by reacting a volatile chloride that he believed to be yttrium chloride with potassium.
In 1843, Carl Gustaf Mosander found that samples of yttria contained three oxides: white yttrium oxide (yttria), yellow terbium oxide (confusingly, this was called 'erbia' at the time) and rose-colored erbium oxide (called 'terbia' at the time). A fourth oxide, ytterbium oxide, was isolated in 1878 by Jean Charles Galissard de Marignac. New elements were later isolated from each of those oxides, and each element was named, in some fashion, after Ytterby, the village near the quarry where they were found (see ytterbium, terbium, and erbium
Erbium is a chemical element with the symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare-earth element, ...
). In the following decades, seven other new metals were discovered in "Gadolin's yttria". Since yttria was found to be a mineral and not an oxide, Martin Heinrich Klaproth renamed it gadolinite
Gadolinite, sometimes known as ytterbite, is a silicate mineral consisting principally of the silicates of cerium, lanthanum, neodymium, yttrium, beryllium, and iron with the formula . It is called gadolinite-(Ce) or gadolinite-(Y), depending o ...
in honor of Gadolin.
Until the early 1920s, the chemical symbol Yt was used for the element, after which Y came into common use.
In 1987, yttrium barium copper oxide was found to achieve high-temperature superconductivity. It was only the second material known to exhibit this property, and it was the first-known material to achieve superconductivity
Superconductivity is a set of physical properties observed in certain materials where electrical resistance vanishes and magnetic flux fields are expelled from the material. Any material exhibiting these properties is a superconductor. Unlike ...
above the (economically important) boiling point of nitrogen.
Occurrence

Abundance
Yttrium is found in mostrare-earth mineral
A rare-earth mineral contains one or more rare-earth elements as major metal constituents. Rare-earth minerals are usually found in association with alkaline to peralkaline igneous complexes, in pegmatites associated with alkaline magmas and ...
s, it is found in some uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
ores, but is never found in the Earth's crust as a free element. About 31 ppm of the Earth's crust is yttrium, making it the 28th most abundant element, 400 times more common than silver
Silver is a chemical element with the symbol Ag (from the Latin ', derived from the Proto-Indo-European ''h₂erǵ'': "shiny" or "white") and atomic number 47. A soft, white, lustrous transition metal, it exhibits the highest electrical ...
. Yttrium is found in soil in concentrations between 10 and 150 ppm (dry weight average of 23 ppm) and in sea water at 9 ppt. Lunar rock samples collected during the American
American(s) may refer to:
* American, something of, from, or related to the United States of America, commonly known as the "United States" or "America"
** Americans, citizens and nationals of the United States of America
** American ancestry, pe ...
Apollo Project have a relatively high content of yttrium. Stwertka 1998, p. 115.
Yttrium has no known biological role, though it is found in most, if not all, organisms and tends to concentrate in the liver, kidney, spleen, lungs, and bones of humans. Normally, as little as is found in the entire human body; human breast milk
Breast milk (sometimes spelled as breastmilk) or mother's milk is milk produced by mammary glands located in the breast of a human female. Breast milk is the primary source of nutrition for newborns, containing fat, protein, carbohydrates ( la ...
contains 4 ppm. Yttrium can be found in edible plants in concentrations between 20 ppm and 100 ppm (fresh weight), with cabbage
Cabbage, comprising several cultivars of ''Brassica oleracea'', is a leafy green, red (purple), or white (pale green) biennial plant grown as an annual vegetable crop for its dense-leaved heads. It is descended from the wild cabbage ( ''B.&n ...
having the largest amount. With as much as 700 ppm, the seeds of woody plants have the highest known concentrations.
there are reports of the discovery of very large reserves of rare-earth elements on a tiny Japanese island. Minami-Torishima Island, also known as Marcus Island, is described as having "tremendous potential" for rare-earth elements and yttrium (REY), according to a study published in Scientific Reports.
"This REY-rich mud has great potential as a rare-earth metal resource because of the enormous amount available and its advantageous mineralogical features," the study reads. The study shows that more than of rare-earth elements could be "exploited in the near future." Including yttrium (Y), which is used in products like camera lenses and mobile phone screens, the rare-earth elements found are europium (Eu), terbium (Tb), and dysprosium (Dy).
Production
As yttrium is chemically similar to lanthanides, it occurs in the same ores (rare-earth mineral
A rare-earth mineral contains one or more rare-earth elements as major metal constituents. Rare-earth minerals are usually found in association with alkaline to peralkaline igneous complexes, in pegmatites associated with alkaline magmas and ...
s) and is extracted by the same refinement processes. A slight distinction is recognized between the light (LREE) and the heavy rare-earth elements (HREE), but the distinction is not perfect. Yttrium is concentrated in the HREE group because of its ion size, though it has a lower atomic mass
The atomic mass (''m''a or ''m'') is the mass of an atom. Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da) – equivalently, unified atomic mass unit (u). 1&n ...
.
 Rare-earth elements (REEs) come mainly from four sources:
* Carbonate and fluoride containing ores such as the LREE bastnäsite (
Rare-earth elements (REEs) come mainly from four sources:
* Carbonate and fluoride containing ores such as the LREE bastnäsite (Mountain Pass rare earth mine
The Mountain Pass Mine, owned by MP Materials, is an open-pit mine of rare-earth elements on the south flank of the Clark Mountain Range in California, southwest of Las Vegas, Nevada. In 2020 the mine supplied 15.8% of the world's rare-earth ...
in California, making the United States the largest producer of REEs during that period. The name "bastnäsite" is actually a group name, and the Levinson suffix is used in the correct mineral names, e.g., bästnasite-(Y) has Y as a prevailing element.
* Monazite (Xenotime
Xenotime is a rare-earth phosphate mineral, the major component of which is yttrium orthophosphate ( Y P O4). It forms a solid solution series with chernovite-(Y) ( Y As O4) and therefore may contain trace impurities of arsenic, as well as sili ...
, a REE phosphate, is the main HREE ore containing as much as 60% yttrium as yttrium phosphate (YPO4). This applies to xenotime-(Y). The largest mine is the Bayan Obo deposit in China, making China the largest exporter for HREE since the closure of the Mountain Pass mine in the 1990s.
* Ion absorption clays or Lognan clays are the weathering products of granite and contain only 1% of REEs. The final ore concentrate can contain as much as 8% yttrium. Ion absorption clays are mostly in southern China. Yttrium is also found in samarskite and fergusonite (which also stand for group names). Emsley 2001, p. 497
One method for obtaining pure yttrium from the mixed oxide ores is to dissolve the oxide in sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
and fractionate it by ion exchange
Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, ...
chromatography
In chemical analysis, chromatography is a laboratory technique for the separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it through a system ( ...
. With the addition of oxalic acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and formula . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name comes from the fact that early invest ...
, the yttrium oxalate precipitates. The oxalate is converted into the oxide by heating under oxygen. By reacting the resulting yttrium oxide with hydrogen fluoride
Hydrogen fluoride (fluorane) is an inorganic compound with the chemical formula . This colorless gas or liquid is the principal industrial source of fluorine, often as an aqueous solution called hydrofluoric acid. It is an important feedstock ...
, yttrium fluoride is obtained. When quaternary ammonium salts are used as extractants, most yttrium will remain in the aqueous phase. When the counter-ion is nitrate, the light lanthanides are removed, and when the counter-ion is thiocyanate, the heavy lanthanides are removed. In this way, yttrium salts of 99.999% purity are obtained. In the usual situation, where yttrium is in a mixture that is two-thirds heavy-lanthanide, yttrium should be removed as soon as possible to facilitate the separation of the remaining elements.
Annual world production of yttrium oxide had reached by 2001; by 2014 it had increased to . Global reserves of yttrium oxide were estimated in 2014 to be more than . The leading countries for these reserves included Australia, Brazil, China, India, and the United States. Only a few tonnes of yttrium metal are produced each year by reducing yttrium fluoride to a metal sponge
Regular foamed aluminium
A metal foam is a cellular structure consisting of a solid metal (frequently aluminium) with gas-filled pores comprising a large portion of the volume. The pores can be sealed (closed-cell foam) or interconnected (open-c ...
with calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar ...
magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
alloy. The temperature of an arc furnace of greater than 1,600 °C is sufficient to melt the yttrium.
Applications
Consumer
 The red component of
The red component of color television
Color television or Colour television is a television transmission technology that includes color information for the picture, so the video image can be displayed in color on the television set. It improves on the monochrome or black-and-white t ...
cathode ray tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms ( oscilloscope), ...
s is typically emitted from an yttria () or yttrium oxide sulfide () host lattice doped with europium (III) cation (Eu3+) phosphor
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or v ...
s. The red color itself is emitted from the europium while the yttrium collects energy from the electron gun and passes it to the phosphor. Daane 1968, p. 818 Yttrium compounds can serve as host lattices for doping with different lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises the 15 metallic chemical elements with atomic numbers 57–71, from lanthanum through lutetium. These elements, along with the chemically similar elements scandium and yt ...
cations. Tb3+ can be used as a doping agent to produce green luminescence
Luminescence is spontaneous emission of light by a substance not resulting from heat; or "cold light".
It is thus a form of cold-body radiation. It can be caused by chemical reactions, electrical energy, subatomic motions or stress on a crys ...
. As such yttrium compounds such as yttrium aluminium garnet (YAG) are useful for phosphors and are an important component of white LEDs.
Yttria is used as a sintering
Clinker nodules produced by sintering
Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction.
Sintering happens as part of a manufacturing ...
additive in the production of porous silicon nitride
Silicon nitride is a chemical compound of the elements silicon and nitrogen. is the most thermodynamically stable and commercially important of the silicon nitrides, and the term "silicon nitride" commonly refers to this specific composition. It ...
.
Yttrium compounds are used as a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
for ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon-carbon double bonds).
Ethylene ...
polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many f ...
. As a metal, yttrium is used on the electrodes of some high-performance spark plugs
A spark plug (sometimes, in British English, a sparking plug, and, colloquially, a plug) is a device for delivering electric current from an ignition system to the combustion chamber of a spark-ignition engine to ignite the compressed fuel/air ...
. Yttrium is used in gas mantles for propane
Propane () is a three-carbon alkane with the molecular formula . It is a gas at standard temperature and pressure, but compressible to a transportable liquid. A by-product of natural gas processing and petroleum refining, it is commonly used as ...
lantern
A lantern is an often portable source of lighting, typically featuring a protective enclosure for the light sourcehistorically usually a candle or a oil lamp, wick in oil, and often a battery-powered light in modern timesto make it easier to ca ...
s as a replacement for thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
, which is radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
.
Currently under development is yttrium-stabilized zirconia as a solid electrolyte and as an oxygen sensor
An oxygen sensor (or lambda sensor, where lambda refers to air–fuel ratio#Air–fuel equivalence ratio (λ), air–fuel equivalence ratio, usually denoted by λ) or probe or wikt:sond, sond, is an electronics, electronic device that measures th ...
in automobile exhaust systems.
Garnets
 Yttrium is used in the production of a large variety of synthetic garnets, and yttria is used to make yttrium iron garnets (, also "YIG"), which are very effective
Yttrium is used in the production of a large variety of synthetic garnets, and yttria is used to make yttrium iron garnets (, also "YIG"), which are very effective microwave
Microwave is a form of electromagnetic radiation with wavelengths ranging from about one meter to one millimeter corresponding to frequencies between 300 MHz and 300 GHz respectively. Different sources define different frequency ra ...
filters
Filter, filtering or filters may refer to:
Science and technology
Computing
* Filter (higher-order function), in functional programming
* Filter (software), a computer program to process a data stream
* Filter (video), a software component that ...
which were recently shown to have magnetic interactions more complex and longer-ranged than understood over the previous four decades. Yttrium, iron
Iron () is a chemical element with symbol Fe (from la, ferrum) and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, right in ...
, aluminium
Aluminium (aluminum in American and Canadian English) is a chemical element with the symbol Al and atomic number 13. Aluminium has a density lower than those of other common metals, at approximately one third that of steel. It ha ...
, and gadolinium garnets (e.g. and ) have important magnetic
Magnetism is the class of physical attributes that are mediated by a magnetic field, which refers to the capacity to induce attractive and repulsive phenomena in other entities. Electric currents and the magnetic moments of elementary particl ...
properties. YIG is also very efficient as an acoustic energy transmitter and transducer. Yttrium aluminium garnet ( or YAG) has a hardness
In materials science, hardness (antonym: softness) is a measure of the resistance to localized plastic deformation induced by either mechanical indentation or abrasion. In general, different materials differ in their hardness; for example hard ...
of 8.5 and is also used as a gemstone
A gemstone (also called a fine gem, jewel, precious stone, or semiprecious stone) is a piece of mineral crystal which, in cut and polished form, is used to make jewelry or other adornments. However, certain rocks (such as lapis lazuli, opal, ...
in jewelry (simulated diamond
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Another solid form of carbon known as graphite is the chemically stable form of carbon at room temperature and pressure, b ...
). Cerium
Cerium is a chemical element with the symbol Ce and atomic number 58. Cerium is a soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it often shows the +3 ...
-doped yttrium aluminium garnet (YAG:Ce) crystals are used as phosphors to make white LEDs.
YAG, yttria, yttrium lithium fluoride Yttrium lithium fluoride (LiYF4, sometimes abbreviated YLF) is a birefringent crystal, typically doped with neodymium or praseodymium and used as a
gain medium in solid-state lasers. Yttrium is the substitutional element in LiYF4. The hardness of ...
(), and yttrium orthovanadate () are used in combination with dopant
A dopant, also called a doping agent, is a trace of impurity element that is introduced into a chemical material to alter its original electrical or optical properties. The amount of dopant necessary to cause changes is typically very low. Whe ...
s such as neodymium
Neodymium is a chemical element with the symbol Nd and atomic number 60. It is the fourth member of the lanthanide series and is considered to be one of the rare-earth metals. It is a hard, slightly malleable, silvery metal that quickly tarn ...
, erbium
Erbium is a chemical element with the symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare-earth element, ...
, ytterbium in near-infrared
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of Light, visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from ...
laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word "laser" is an acronym for "light amplification by stimulated emission of radiation". The ...
s. YAG lasers can operate at high power and are used for drilling and cutting metal. The single crystals of doped YAG are normally produced by the Czochralski process.
Material enhancer
Small amounts of yttrium (0.1 to 0.2%) have been used to reduce the grain sizes ofchromium
Chromium is a chemical element with the symbol Cr and atomic number 24. It is the first element in group 6. It is a steely-grey, lustrous, hard, and brittle transition metal.
Chromium metal is valued for its high corrosion resistance and hard ...
, molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lead ...
, titanium
Titanium is a chemical element with the symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion i ...
, and zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'' ...
. Yttrium is used to increase the strength of aluminium and magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic ...
alloys. The addition of yttrium to alloys generally improves workability, adds resistance to high-temperature recrystallization, and significantly enhances resistance to high-temperature oxidation
Redox (reduction–oxidation, , ) is a type of chemical reaction in which the oxidation states of substrate change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or ...
(see graphite nodule discussion below).
Yttrium can be used to deoxidize vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( pass ...
and other non-ferrous metal
In metallurgy, non-ferrous metals are metals or alloys that do not contain iron ( allotropes of iron, ferrite, and so on) in appreciable amounts.
Generally more costly than ferrous metals, non-ferrous metals are used because of desirable prop ...
s. Yttria stabilizes the cubic form of zirconia in jewelry.
Yttrium has been studied as a nodulizer in ductile cast iron, forming the graphite
Graphite () is a crystalline form of the element carbon. It consists of stacked layers of graphene. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on la ...
into compact nodules instead of flakes to increase ductility
Ductility is a mechanical property commonly described as a material's amenability to drawing (e.g. into wire). In materials science, ductility is defined by the degree to which a material can sustain plastic deformation under tensile str ...
and fatigue resistance. Having a high melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state from solid to liquid. At the melting point the solid and liquid phase exist in equilibrium. The melting point of a substance depen ...
, yttrium oxide is used in some ceramic
A ceramic is any of the various hard, brittle, heat-resistant and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcelain, ...
and glass
Glass is a non- crystalline, often transparent, amorphous solid that has widespread practical, technological, and decorative use in, for example, window panes, tableware, and optics. Glass is most often formed by rapid cooling (quenchin ...
to impart shock
Shock may refer to:
Common uses Collective noun
*Shock, a historic commercial term for a group of 60, see English numerals#Special names
* Stook, or shock of grain, stacked sheaves
Healthcare
* Shock (circulatory), circulatory medical emerge ...
resistance and low thermal expansion
Thermal expansion is the tendency of matter to change its shape, area, volume, and density in response to a change in temperature, usually not including phase transitions.
Temperature is a monotonic function of the average molecular kin ...
properties. Those same properties make such glass useful in camera lens
A camera lens (also known as photographic lens or photographic objective) is an optical lens or assembly of lenses used in conjunction with a camera body and mechanism to make images of objects either on photographic film or on other media cap ...
es.
Medical
The radioactive isotope yttrium-90 is used in drugs such asYttrium Y 90-DOTA-tyr3-octreotide
Edotreotide (USAN, also known as (DOTA0- Phe1- Tyr3) octreotide, DOTA-TOC, DOTATOC) is a substance which, when bound to various radionuclides, is used in the treatment and diagnosis of certain types of cancer. When used therapeutically it is an e ...
and Yttrium Y 90 ibritumomab tiuxetan for the treatment of various cancer
Cancer is a group of diseases involving abnormal cell growth with the potential to invade or spread to other parts of the body. These contrast with benign tumors, which do not spread. Possible signs and symptoms include a lump, abnormal b ...
s, including lymphoma
Lymphoma is a group of blood and lymph tumors that develop from lymphocytes (a type of white blood cell). In current usage the name usually refers to just the cancerous versions rather than all such tumours. Signs and symptoms may include en ...
, leukemia
Leukemia ( also spelled leukaemia and pronounced ) is a group of blood cancers that usually begin in the bone marrow and result in high numbers of abnormal blood cells. These blood cells are not fully developed and are called ''blasts'' or ...
, liver, ovarian, colorectal, pancreatic and bone cancers. Emsley 2001, p. 495 It works by adhering to monoclonal antibodies
A monoclonal antibody (mAb, more rarely called moAb) is an antibody produced from a cell Lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell.
Monoclonal antibodies ...
, which in turn bind to cancer cells and kill them via intense β-radiation from the yttrium-90 (see monoclonal antibody therapy
Monoclonal antibody therapy is a form of immunotherapy that uses monoclonal antibodies (mAbs) to bind monospecifically to certain cells or proteins. The objective is that this treatment will stimulate the patient's immune system to attack tho ...
).
A technique called radioembolization is used to treat hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer in adults and is currently the most common cause of death in people with cirrhosis. HCC is the third leading cause of cancer-related deaths worldwide.
It occurs in t ...
and liver metastasis. Radioembolization is a low toxicity, targeted liver cancer therapy that uses millions of tiny beads made of glass or resin containing radioactive yttrium-90. The radioactive microspheres are delivered directly to the blood vessels feeding specific liver tumors/segments or lobes. It is minimally invasive and patients can usually be discharged after a few hours. This procedure may not eliminate all tumors throughout the entire liver, but works on one segment or one lobe at a time and may require multiple procedures.
Also see radioembolization in the case of combined cirrhosis and hepatocellular carcinoma.
Needles made of yttrium-90, which can cut more precisely than scalpels, have been used to sever pain-transmitting nerve
A nerve is an enclosed, cable-like bundle of nerve fibers (called axons) in the peripheral nervous system.
A nerve transmits electrical impulses. It is the basic unit of the peripheral nervous system. A nerve provides a common pathway for the ...
s in the spinal cord
The spinal cord is a long, thin, tubular structure made up of nervous tissue, which extends from the medulla oblongata in the brainstem to the lumbar region of the vertebral column (backbone). The backbone encloses the central canal of the sp ...
, and yttrium-90 is also used to carry out radionuclide synovectomy in the treatment of inflamed joints, especially knees, in sufferers of conditions such as rheumatoid arthritis
Rheumatoid arthritis (RA) is a long-term autoimmune disorder that primarily affects joints. It typically results in warm, swollen, and painful joints. Pain and stiffness often worsen following rest. Most commonly, the wrist and hands are inv ...
.
A neodymium-doped yttrium-aluminium-garnet laser has been used in an experimental, robot-assisted radical prostatectomy in canines in an attempt to reduce collateral nerve and tissue damage, and erbium-doped lasers are coming into use for cosmetic skin resurfacing.
Superconductors
 Yttrium is a key ingredient in the yttrium barium copper oxide (YBa2Cu3O7, aka 'YBCO' or '1-2-3') superconductor developed at the
Yttrium is a key ingredient in the yttrium barium copper oxide (YBa2Cu3O7, aka 'YBCO' or '1-2-3') superconductor developed at the University of Alabama
The University of Alabama (informally known as Alabama, UA, or Bama) is a public research university in Tuscaloosa, Alabama. Established in 1820 and opened to students in 1831, the University of Alabama is the oldest and largest of the publ ...
and the University of Houston
The University of Houston (UH) is a Public university, public research university in Houston, Texas. Founded in 1927, UH is a member of the University of Houston System and the List of universities in Texas by enrollment, university in Texas ...
in 1987. This superconductor is notable because the operating superconductivity temperature is above liquid nitrogen
Liquid nitrogen—LN2—is nitrogen in a liquid state at low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, low viscosity liquid that is wid ...
's boiling point (77.1 K). Since liquid nitrogen is less expensive than the liquid helium required for metallic superconductors, the operating costs for applications would be less.
The actual superconducting material is often written as YBa2Cu3O7–''d'', where ''d'' must be less than 0.7 for superconductivity. The reason for this is still not clear, but it is known that the vacancies occur only in certain places in the crystal, the copper oxide planes, and chains, giving rise to a peculiar oxidation state of the copper atoms, which somehow leads to the superconducting behavior.
The theory of low temperature superconductivity has been well understood since the BCS theory of 1957. It is based on a peculiarity of the interaction between two electrons in a crystal lattice. However, the BCS theory does not explain high temperature superconductivity, and its precise mechanism is still a mystery. What is known is that the composition of the copper-oxide materials must be precisely controlled for superconductivity to occur.
This superconductor is a black and green, multi-crystal, multi-phase mineral. Researchers are studying a class of materials known as perovskite
Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula ). Its name is also applied to the class of compounds which have the same type of crystal structure as (XIIA2+VIB4+X2−3), known a ...
s that are alternative combinations of these elements, hoping to develop a practical high-temperature superconductor.
Lithium batteries
Yttrium is used in small quantities in cathodes of someLithium iron phosphate battery
The lithium iron phosphate battery (LFP (lithium ferro-phosphate), or Li-IP) is a type of lithium-ion battery using lithium iron phosphate () as the cathode material, and a graphitic carbon electrode with a metallic backing as the anode.
Becaus ...
(LFP), and then called commonly LiFeYPO4 chemistry, or LYP. Similar to LFP, LYP batteries offer high energy density
In physics, energy density is the amount of energy stored in a given system or region of space per unit volume. It is sometimes confused with energy per unit mass which is properly called specific energy or .
Often only the ''useful'' or extrac ...
, good safety and long life. But LYP, offer higher cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in whi ...
stability, and prolong life of battery, by protecting physical structure of the cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. A conventional current describes the direction in whi ...
, especially at higher temperatures and higher charging / discharge current. LYP batteries do find use in stationary applications ( off-grid solar systems), electric vehicles
An electric vehicle (EV) is a vehicle that uses one or more electric motors for propulsion. It can be powered by a collector system, with electricity from extravehicular sources, or it can be powered autonomously by a battery (sometimes ch ...
(some cars), as well other applications (submarines, ships), similar to LFP batteries, but often at improved safety and cycle life timeLYP cells
have essentially same
nominal voltage
Nominal may refer to:
Linguistics and grammar
* Nominal (linguistics), one of the parts of speech
* Nominal, the adjectival form of "noun", as in "nominal agreement" (= "noun agreement")
* Nominal sentence, a sentence without a finite verb
* ...
as LFP, of 3.25V, but the maximum charging voltage is 4.0V, and very similar charging and discharge characteristic.
Other applications
In 2009, ProfessorMas Subramanian
Mas Subramanian, (born 1954), is a solid-state materials scientist at Oregon State University in Corvallis, Oregon. He is a University Distinguished Professor and the Milton Harris Chair of Materials Science in the university's Department of ...
and associates at Oregon State University
Oregon State University (OSU) is a public land-grant, research university in Corvallis, Oregon. OSU offers more than 200 undergraduate-degree programs along with a variety of graduate and doctoral degrees. It has the 10th largest engineering ...
discovered that yttrium can be combined with indium and manganese
Manganese is a chemical element with the Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of ...
to form an intensely blue
Blue is one of the three primary colours in the RYB colour model (traditional colour theory), as well as in the RGB (additive) colour model. It lies between violet and cyan on the spectrum of visible light. The eye perceives blue when ...
, non-toxic, inert, fade-resistant pigment
A pigment is a colored material that is completely or nearly insoluble in water. In contrast, dyes are typically soluble, at least at some stage in their use. Generally dyes are often organic compounds whereas pigments are often inorganic compou ...
, YInMn blue, the first new blue pigment discovered in 200 years.
Precautions
Yttrium currently has no known biological role, and it can be highlytoxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a sub ...
to humans, animals and plants.
Water-soluble compounds of yttrium are considered mildly toxic, while its insoluble compounds are non-toxic. In experiments on animals, yttrium and its compounds caused lung and liver damage, though toxicity varies with different yttrium compounds. In rats, inhalation of yttrium citrate caused pulmonary edema
Pulmonary edema, also known as pulmonary congestion, is excessive liquid accumulation in the tissue and air spaces (usually alveoli) of the lungs. It leads to impaired gas exchange and may cause hypoxemia and respiratory failure. It is due ...
and dyspnea
Shortness of breath (SOB), also medically known as dyspnea (in AmE) or dyspnoea (in BrE), is an uncomfortable feeling of not being able to breathe well enough. The American Thoracic Society defines it as "a subjective experience of breathing di ...
, while inhalation of yttrium chloride caused liver edema, pleural effusion
A pleural effusion is accumulation of excessive fluid in the pleural space, the potential space that surrounds each lung.
Under normal conditions, pleural fluid is secreted by the parietal pleural capillaries at a rate of 0.6 millilitre per k ...
s, and pulmonary hyperemia. (public domain text)
Exposure to yttrium compounds in humans may cause lung disease. Workers exposed to airborne yttrium europium vanadate dust experienced mild eye, skin, and upper respiratory tract irritation—though this may be caused by the vanadium
Vanadium is a chemical element with the symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer ( pass ...
content rather than the yttrium. Acute exposure to yttrium compounds can cause shortness of breath, coughing, chest pain, and cyanosis
Cyanosis is the change of body tissue color to a bluish-purple hue as a result of having decreased amounts of oxygen bound to the hemoglobin in the red blood cells of the capillary bed. Body tissues that show cyanosis are usually in locations ...
. The Occupational Safety and Health Administration
The Occupational Safety and Health Administration'' (OSHA ) is a large regulatory agency of the United States Department of Labor that originally had federal visitorial powers to inspect and examine workplaces. Congress established the agen ...
(OSHA) limits exposure to yttrium in the workplace to over an 8-hour workday. The National Institute for Occupational Safety and Health
The National Institute for Occupational Safety and Health (NIOSH, ) is the United States federal agency responsible for conducting research and making recommendations for the prevention of work-related injury and illness. NIOSH is part of the C ...
(NIOSH) recommended exposure limit (REL) is over an 8-hour workday. At levels of , yttrium is immediately dangerous to life and health. Yttrium dust is highly flammable.
See also
*Notes
References
Bibliography
* * * * * * *Further reading
* *External links
Yttrium by Paul C.W. Chu at acs.org
at '' The Periodic Table of Videos'' (University of Nottingham) *
Encyclopedia of Geochemistry - Yttrium
{{Authority control Chemical elements Transition metals Deoxidizers Chemical elements with hexagonal close-packed structure