Technetium on:
[Wikipedia]
[Google]
[Amazon]
Technetium is a
 The most prevalent form of technetium that is easily accessible is sodium pertechnetate, Na cO4 The majority of this material is produced by radioactive decay from sup>99MoO4sup>2−:
: sup>99MoO4sup>2− → sup>99mTcO4sup>− + e−
Pertechnetate (tetroxidotechnetate) behaves analogously to perchlorate, both of which are tetrahedral. Unlike
The most prevalent form of technetium that is easily accessible is sodium pertechnetate, Na cO4 The majority of this material is produced by radioactive decay from sup>99MoO4sup>2−:
: sup>99MoO4sup>2− → sup>99mTcO4sup>− + e−
Pertechnetate (tetroxidotechnetate) behaves analogously to perchlorate, both of which are tetrahedral. Unlike
 The following binary (containing only two elements) technetium halides are known: TcF6, TcF5, TcCl4, TcBr4, TcBr3, α-TcCl3, β-TcCl3, TcI3, α-TcCl2, and β-TcCl2. The
The following binary (containing only two elements) technetium halides are known: TcF6, TcF5, TcCl4, TcBr4, TcBr3, α-TcCl3, β-TcCl3, TcI3, α-TcCl2, and β-TcCl2. The  Two polymorphs of technetium trichloride exist, α- and β-TcCl3. The α polymorph is also denoted as Tc3Cl9. It adopts a confacial bioctahedral structure. It is prepared by treating the chloro-acetate Tc2(O2CCH3)4Cl2 with HCl. Like Re3Cl9, the structure of the α-polymorph consists of triangles with short M-M distances. β-TcCl3 features octahedral Tc centers, which are organized in pairs, as seen also for molybdenum trichloride. TcBr3 does not adopt the structure of either trichloride phase. Instead it has the structure of molybdenum tribromide, consisting of chains of confacial octahedra with alternating short and long Tc—Tc contacts. TcI3 has the same structure as the high temperature phase of TiI3, featuring chains of confacial octahedra with equal Tc—Tc contacts.
Several anionic technetium halides are known. The binary tetrahalides can be converted to the hexahalides cX6sup>2− (X = F, Cl, Br, I), which adopt
Two polymorphs of technetium trichloride exist, α- and β-TcCl3. The α polymorph is also denoted as Tc3Cl9. It adopts a confacial bioctahedral structure. It is prepared by treating the chloro-acetate Tc2(O2CCH3)4Cl2 with HCl. Like Re3Cl9, the structure of the α-polymorph consists of triangles with short M-M distances. β-TcCl3 features octahedral Tc centers, which are organized in pairs, as seen also for molybdenum trichloride. TcBr3 does not adopt the structure of either trichloride phase. Instead it has the structure of molybdenum tribromide, consisting of chains of confacial octahedra with alternating short and long Tc—Tc contacts. TcI3 has the same structure as the high temperature phase of TiI3, featuring chains of confacial octahedra with equal Tc—Tc contacts.
Several anionic technetium halides are known. The binary tetrahalides can be converted to the hexahalides cX6sup>2− (X = F, Cl, Br, I), which adopt
 Technetium forms a variety of
Technetium forms a variety of
^_U -> ce^_I + ^_Y + 2^_n
: ^_Y -> beta^-1.47\,\ce] ^_Zr -> beta^-2.1\,\ce] ^_Nb -> beta^-15.0\,\ce] ^_Mo -> beta^-65.94\,\ce] ^_Tc -> beta^-211,100\,\ce] ^_Ru
Because used fuel is allowed to stand for several years before reprocessing, all molybdenum-99 and technetium-99m is decayed by the time that the fission products are separated from the major  Almost two-thirds of the world's supply comes from two reactors; the National Research Universal Reactor at
Almost two-thirds of the world's supply comes from two reactors; the National Research Universal Reactor at
EnvironmentalChemistry.com – Technetium
!--per the guidelines a
Wikipedia's WikiProject Elements
(all viewed 1 December 2002)-->
Nudat 2
nuclide chart from the National Nuclear Data Center, Brookhaven National Laboratory
at '' The Periodic Table of Videos'' (University of Nottingham) {{Authority control Chemical elements Transition metals Synthetic elements Radiopharmaceuticals Chemical elements predicted by Dmitri Mendeleev Chemical elements with hexagonal close-packed structure
chemical element
A chemical element is a species of atoms that have a given number of protons in their atomic nucleus, nuclei, including the pure Chemical substance, substance consisting only of that species. Unlike chemical compounds, chemical elements canno ...
with the symbol
A symbol is a mark, sign, or word that indicates, signifies, or is understood as representing an idea, object, or relationship. Symbols allow people to go beyond what is known or seen by creating linkages between otherwise very different conc ...
Tc and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
43. It is the lightest element whose isotopes are all radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
. All available technetium is produced as a synthetic element
A synthetic element is one of 24 known chemical elements that do not occur naturally on Earth: they have been created by human manipulation of fundamental particles in a nuclear reactor, a particle accelerator, or the explosion of an atomic bomb; ...
. Naturally occurring technetium is a spontaneous fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
in uranium ore and thorium
Thorium is a weakly radioactive metallic chemical element with the symbol Th and atomic number 90. Thorium is silvery and tarnishes black when it is exposed to air, forming thorium dioxide; it is moderately soft and malleable and has a high ...
ore, the most common source, or the product of neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons ...
in molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lead ...
ores. This silvery gray, crystalline transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that can ...
lies between manganese
Manganese is a chemical element with the Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of ...
and rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
in group 7 of the periodic table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ch ...
, and its chemical properties are intermediate between those of both adjacent elements. The most common naturally occurring isotope is 99Tc, in traces only.
Many of technetium's properties had been predicted by Dmitri Mendeleev
Dmitri Ivanovich Mendeleev (sometimes transliterated as Mendeleyev or Mendeleef) ( ; russian: links=no, Дмитрий Иванович Менделеев, tr. , ; 8 February Old_Style_and_New_Style_dates">O.S._27_January.html" ;"title="O ...
before it was discovered. Mendeleev noted a gap in his periodic table and gave the undiscovered element the provisional name '' ekamanganese'' (''Em''). In 1937, technetium (specifically the technetium-97 isotope) became the first predominantly artificial element to be produced, hence its name (from the Greek , '' technetos'', from ''techne'', as in "craft", "art" and having the meaning of "artificial", +
One short-lived gamma ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically ...
-emitting nuclear isomer, technetium-99m
Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99 (itself an isotope of technetium), symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used medica ...
, is used in nuclear medicine
Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is " radiology done inside out" because it records radiation emi ...
for a wide variety of tests, such as bone cancer diagnoses. The ground state of the nuclide
A nuclide (or nucleide, from atomic nucleus, nucleus, also known as nuclear species) is a class of atoms characterized by their number of protons, ''Z'', their number of neutrons, ''N'', and their nuclear energy state.
The word ''nuclide'' was co ...
technetium-99 is used as a gamma-ray-free source of beta particle
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, � ...
s. Long-lived technetium isotopes produced commercially are byproducts of the fission of uranium-235
Uranium-235 (235U or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exi ...
in nuclear reactors
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat from ...
and are extracted from nuclear fuel rods. Because even the longest-lived isotope of technetium has a relatively short half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
(4.21 million years), the 1952 detection of technetium in red giant
A red giant is a luminous giant star of low or intermediate mass (roughly 0.3–8 solar masses ()) in a late phase of stellar evolution. The outer atmosphere is inflated and tenuous, making the radius large and the surface temperature around o ...
s helped to prove that stars can produce heavier elements.
History
Search for element 43
From the 1860s through 1871, early forms of the periodic table proposed byDmitri Mendeleev
Dmitri Ivanovich Mendeleev (sometimes transliterated as Mendeleyev or Mendeleef) ( ; russian: links=no, Дмитрий Иванович Менделеев, tr. , ; 8 February Old_Style_and_New_Style_dates">O.S._27_January.html" ;"title="O ...
contained a gap between molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lead ...
(element 42) and ruthenium
Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemical ...
(element 44). In 1871, Mendeleev predicted this missing element would occupy the empty place below manganese
Manganese is a chemical element with the Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of ...
and have similar chemical properties. Mendeleev gave it the provisional name ''ekamanganese'' (from ''eka''-, the Sanskrit
Sanskrit (; attributively , ; nominally , , ) is a classical language belonging to the Indo-Aryan languages, Indo-Aryan branch of the Indo-European languages. It arose in South Asia after its predecessor languages had Trans-cultural diffusion ...
word for ''one'') because the predicted element was one place down from the known element manganese.
Early misidentifications
Many early researchers, both before and after the periodic table was published, were eager to be the first to discover and name the missing element. Its location in the table suggested that it should be easier to find than other undiscovered elements.Irreproducible results
German chemistsWalter Noddack
Walter Noddack (17 August 1893 – 7 December 1960) was a German chemist. He, Ida Tacke (who later married Noddack), and Otto Berg reported the discovery of element 43 and element 75 in 1925.
Rhenium
They named element 75 rhenium ( Latin ''R ...
, Otto Berg, and Ida Tacke
Ida Noddack (25 February 1896 – 24 September 1978), ''née'' Tacke, was a German chemist and physicist. In 1934 she was the first to mention the idea later named nuclear fission. With her husband - Walter Noddack - and Otto Berg she discover ...
reported the discovery of element 75 and element 43 in 1925, and named element 43 ''masurium'' (after Masuria in eastern Prussia
Prussia, , Old Prussian: ''Prūsa'' or ''Prūsija'' was a German state on the southeast coast of the Baltic Sea. It formed the German Empire under Prussian rule when it united the German states in 1871. It was ''de facto'' dissolved by an e ...
, now in Poland
Poland, officially the Republic of Poland, is a country in Central Europe. It is divided into 16 administrative provinces called voivodeships, covering an area of . Poland has a population of over 38 million and is the fifth-most populou ...
, the region where Walter Noddack's family originated). The group bombarded columbite
Columbite, also called niobite, niobite-tantalite and columbate [], is a black mineral group that is an ore of niobium. It has a submetallic Lustre (mineralogy), luster and a high density and is a niobate of iron and manganese. This mineral group w ...
with a beam of electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have n ...
s and deduced element 43 was present by examining X-ray
An X-ray, or, much less commonly, X-radiation, is a penetrating form of high-energy electromagnetic radiation. Most X-rays have a wavelength ranging from 10 picometers to 10 nanometers, corresponding to frequencies in the range 30&nb ...
emission spectrograms. The wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tr ...
of the X-rays produced is related to the atomic number by a formula
In science, a formula is a concise way of expressing information symbolically, as in a mathematical formula or a ''chemical formula''. The informal use of the term ''formula'' in science refers to the general construct of a relationship betwe ...
derived by Henry Moseley in 1913. The team claimed to detect a faint X-ray signal at a wavelength produced by element 43. Later experimenters could not replicate the discovery, and it was dismissed as an error for many years. Still, in 1933, a series of articles on the discovery of elements quoted the name ''masurium'' for element 43. Whether the 1925 team actually discovered element 43 is still debated.
Official discovery and later history
Thediscovery
Discovery may refer to:
* Discovery (observation), observing or finding something unknown
* Discovery (fiction), a character's learning something unknown
* Discovery (law), a process in courts of law relating to evidence
Discovery, The Discove ...
of element 43 was finally confirmed in a 1937 experiment at the University of Palermo in Sicily by Carlo Perrier and Emilio Segrè. In mid-1936, Segrè visited the United States, first Columbia University
Columbia University (also known as Columbia, and officially as Columbia University in the City of New York) is a private research university in New York City. Established in 1754 as King's College on the grounds of Trinity Church in Manhatt ...
in New York and then the Lawrence Berkeley National Laboratory
Lawrence Berkeley National Laboratory (LBNL), commonly referred to as the Berkeley Lab, is a United States national laboratory that is owned by, and conducts scientific research on behalf of, the United States Department of Energy. Located in ...
in California. He persuaded cyclotron
A cyclotron is a type of particle accelerator invented by Ernest O. Lawrence in 1929–1930 at the University of California, Berkeley, and patented in 1932. Lawrence, Ernest O. ''Method and apparatus for the acceleration of ions'', filed: Jan ...
inventor Ernest Lawrence
Ernest Orlando Lawrence (August 8, 1901 – August 27, 1958) was an American nuclear physicist and winner of the Nobel Prize in Physics in 1939 for his invention of the cyclotron. He is known for his work on uranium-isotope separation fo ...
to let him take back some discarded cyclotron parts that had become radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
. Lawrence mailed him a molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lead ...
foil that had been part of the deflector in the cyclotron.
Segrè enlisted his colleague Perrier to attempt to prove, through comparative chemistry, that the molybdenum activity was indeed from an element with the atomic number 43. In 1937, they succeeded in isolating the isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
s technetium-95m and technetium-97. University of Palermo officials wanted them to name their discovery "''panormium''", after the Latin
Latin (, or , ) is a classical language belonging to the Italic languages, Italic branch of the Indo-European languages. Latin was originally a dialect spoken in the lower Tiber area (then known as Latium) around present-day Rome, but through ...
name for Palermo
Palermo ( , ; scn, Palermu , locally also or ) is a city in southern Italy, the capital of both the autonomous region of Sicily and the Metropolitan City of Palermo, the city's surrounding metropolitan province. The city is noted for its ...
, ''Panormus''. In 1947 element 43 was named after the Greek
Greek may refer to:
Greece
Anything of, from, or related to Greece, a country in Southern Europe:
*Greeks, an ethnic group.
*Greek language, a branch of the Indo-European language family.
**Proto-Greek language, the assumed last common ancestor ...
word ''τεχνητός'', meaning "artificial", since it was the first element to be artificially produced. Segrè returned to Berkeley and met Glenn T. Seaborg. They isolated the metastable isotope technetium-99m
Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99 (itself an isotope of technetium), symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used medica ...
, which is now used in some ten million medical diagnostic procedures annually.
In 1952, the astronomer Paul W. Merrill in California detected the spectral signature of technetium (specifically wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tr ...
s of 403.1 nm, 423.8 nm, 426.2 nm, and 429.7 nm) in light from S-type red giant
A red giant is a luminous giant star of low or intermediate mass (roughly 0.3–8 solar masses ()) in a late phase of stellar evolution. The outer atmosphere is inflated and tenuous, making the radius large and the surface temperature around o ...
s. The stars were near the end of their lives but were rich in the short-lived element, which indicated that it was being produced in the stars by nuclear reaction
In nuclear physics and nuclear chemistry, a nuclear reaction is a process in which two nuclei, or a nucleus and an external subatomic particle, collide to produce one or more new nuclides. Thus, a nuclear reaction must cause a transformatio ...
s. That evidence bolstered the hypothesis that heavier elements are the product of nucleosynthesis in stars. More recently, such observations provided evidence that elements are formed by neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons ...
in the s-process
The slow neutron-capture process, or ''s''-process, is a series of reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The ''s''-process is responsible for the creation ( nucleosynthesis) of approxim ...
.
Since that discovery, there have been many searches in terrestrial materials for natural sources of technetium. In 1962, technetium-99 was isolated and identified in pitchblende from the Belgian Congo
The Belgian Congo (french: Congo belge, ; nl, Belgisch-Congo) was a Belgian colony in Central Africa from 1908 until independence in 1960. The former colony adopted its present name, the Democratic Republic of the Congo (DRC), in 1964.
Colo ...
in extremely small quantities (about 0.2 ng/kg), where it originates as a spontaneous fission
Spontaneous fission (SF) is a form of radioactive decay that is found only in very heavy chemical elements. The nuclear binding energy of the elements reaches its maximum at an atomic mass number of about 56 (e.g., iron-56); spontaneous breakd ...
product of uranium-238
Uranium-238 (238U or U-238) is the most common isotope of uranium found in nature, with a relative abundance of 99%. Unlike uranium-235, it is non-fissile, which means it cannot sustain a chain reaction in a thermal-neutron reactor. However ...
. The Oklo
Oklo is a region near the town of Franceville, in the Haut-Ogooué province of the Central African country of Gabon. Several natural nuclear fission reactors were discovered in the uranium mines in the region in 1972.
History
Gabon was a Frenc ...
natural nuclear fission reactor
A natural nuclear fission reactor is a uranium deposit where self-sustaining nuclear chain reactions occur. The conditions under which a natural nuclear reactor could exist had been predicted in 1956 by Japanese American chemist Paul Kuroda. ...
contains evidence that significant amounts of technetium-99 were produced and have since decayed into ruthenium-99.
Characteristics
Physical properties
Technetium is a silvery-gray radioactivemetal
A metal (from ancient Greek, Greek μέταλλον ''métallon'', "mine, quarry, metal") is a material that, when freshly prepared, polished, or fractured, shows a lustrous appearance, and conducts electrical resistivity and conductivity, e ...
with an appearance similar to platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Pla ...
, commonly obtained as a gray powder. The crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pattern ...
of the bulk pure metal is hexagonal
In geometry, a hexagon (from Greek , , meaning "six", and , , meaning "corner, angle") is a six-sided polygon. The total of the internal angles of any simple (non-self-intersecting) hexagon is 720°.
Regular hexagon
A '' regular hexagon'' has ...
close-packed. The crystal structure
In crystallography, crystal structure is a description of the ordered arrangement of atoms, ions or molecules in a crystalline material. Ordered structures occur from the intrinsic nature of the constituent particles to form symmetric pattern ...
of the nanodisperse pure metal is cubic. Nanodisperse technetium does not have a split NMR spectrum, while hexagonal bulk technetium has the Tc-99-NMR spectrum split in 9 satellites. Atomic technetium has characteristic emission lines
A spectral line is a dark or bright line in an otherwise uniform and continuous spectrum, resulting from emission or absorption of light in a narrow frequency range, compared with the nearby frequencies. Spectral lines are often used to ident ...
at wavelength
In physics, the wavelength is the spatial period of a periodic wave—the distance over which the wave's shape repeats.
It is the distance between consecutive corresponding points of the same phase on the wave, such as two adjacent crests, tr ...
s of 363.3 nm, 403.1 nm, 426.2 nm, 429.7 nm, and 485.3 nm.
The metal form is slightly paramagnetic
Paramagnetism is a form of magnetism whereby some materials are weakly attracted by an externally applied magnetic field, and form internal, induced magnetic fields in the direction of the applied magnetic field. In contrast with this behavior, ...
, meaning its magnetic dipoles align with external magnetic field
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to ...
s, but will assume random orientations once the field is removed. Pure, metallic, single-crystal technetium becomes a type-II superconductor
In superconductivity, a type-II superconductor is a superconductor that exhibits an intermediate phase of mixed ordinary and superconducting properties at intermediate temperature and fields above the superconducting phases.
It also features the ...
at temperatures below 7.46 K. Below this temperature, technetium has a very high magnetic penetration depth, greater than any other element except niobium
Niobium is a chemical element with chemical symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition metal. Pure niobium has a Mohs hardness rating similar to pure titanium, and it has s ...
.
Chemical properties
Technetium is located in the seventh group of the periodic table, betweenrhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
and manganese
Manganese is a chemical element with the Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of ...
. As predicted by the periodic law, its chemical properties are between those two elements. Of the two, technetium more closely resembles rhenium, particularly in its chemical inertness and tendency to form covalent bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between ato ...
s. This is consistent with the tendency of period 5 elements to resemble their counterparts in period 6 more than period 4 due to the lanthanide contraction
The lanthanide contraction is the greater-than-expected decrease in atomic radii/ionic radii of the elements in the lanthanide series from atomic number 57, lanthanum, to 71, lutetium, which results in smaller than otherwise expected atomic radii ...
. Unlike manganese, technetium does not readily form cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
s ( ions with a net positive charge). Technetium exhibits nine oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
s from −1 to +7, with +4, +5, and +7 being the most common. Technetium dissolves in aqua regia, nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
, and concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
, but it is not soluble in hydrochloric acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid. It is a component of the gastric acid in the dige ...
of any concentration.
Metallic technetium slowly tarnishes in moist air and, in powder form, burns in oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements ...
.
Technetium can catalyse the destruction of hydrazine
Hydrazine is an inorganic compound with the chemical formula . It is a simple pnictogen hydride, and is a colourless flammable liquid with an ammonia-like odour. Hydrazine is highly toxic unless handled in solution as, for example, hydrazine ...
by nitric acid
Nitric acid is the inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but older samples tend to be yellow cast due to decomposition into oxides of nitrogen. Most commercially available ni ...
, and this property is due to its multiplicity of valencies. This caused a problem in the separation of plutonium from uranium in nuclear fuel processing, where hydrazine is used as a protective reductant to keep plutonium in the trivalent rather than the more stable tetravalent state. The problem was exacerbated by the mutually enhanced solvent extraction of technetium and zirconium at the previous stage, and required a process modification.
Compounds
Pertechnetate and derivatives
permanganate
A permanganate () is a chemical compound containing the manganate(VII) ion, , the conjugate base of permanganic acid. Because the manganese atom is in the +7 oxidation state, the permanganate(VII) ion is a strong oxidizing agent. The ion is a ...
(), it is only a weak oxidizing agent
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or " accepts"/"receives" an electron from a (called the , , or ). In other words, an oxi ...
.
Related to pertechnetate is technetium heptoxide. This pale-yellow, volatile solid is produced by oxidation of Tc metal and related precursors:
:4 Tc + 7 O2 → 2 Tc2O7
It is a molecular metal oxide, analogous to manganese heptoxide. It adopts a centrosymmetric
In crystallography, a centrosymmetric point group contains an inversion center as one of its symmetry elements. In such a point group, for every point (x, y, z) in the unit cell there is an indistinguishable point (-x, -y, -z). Such point gr ...
structure with two types of Tc−O bonds with 167 and 184 pm bond lengths.
Technetium heptoxide hydrolyzes to pertechnetate and pertechnetic acid, depending on the pH:
:Tc2O7 + 2 OH− → 2 TcO4− + H2O
:Tc2O7 + H2O → 2 HTcO4
HTcO4 is a strong acid. In concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid ( Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen and hydrogen, with the molecular fo ...
, cO4sup>− converts to the octahedral form TcO3(OH)(H2O)2, the conjugate base of the hypothetical triaquo complex
In chemistry, metal aquo complexes are coordination compounds containing metal ions with only water as a ligand. These complexes are the predominant species in aqueous solutions of many metal salts, such as metal nitrates, sulfates, and perchlorat ...
cO3(H2O)3sup>+.
Other chalcogenide derivatives
Technetium forms adioxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of ...
, disulfide
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In ...
, di selenide, and di telluride. An ill-defined Tc2S7 forms upon treating pertechnate with hydrogen sulfide. It thermally decomposes into disulfide and elemental sulfur. Similarly the dioxide can be produced by reduction of the Tc2O7.
Unlike the case for rhenium, a trioxide has not been isolated for technetium. However, TcO3 has been identified in the gas phase using mass spectrometry
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a '' mass spectrum'', a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is u ...
.
Simple hydride and halide complexes
Technetium forms the simple complex . The potassium salt is isostructural with . The following binary (containing only two elements) technetium halides are known: TcF6, TcF5, TcCl4, TcBr4, TcBr3, α-TcCl3, β-TcCl3, TcI3, α-TcCl2, and β-TcCl2. The
The following binary (containing only two elements) technetium halides are known: TcF6, TcF5, TcCl4, TcBr4, TcBr3, α-TcCl3, β-TcCl3, TcI3, α-TcCl2, and β-TcCl2. The oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic. It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound. C ...
s range from Tc(VI) to Tc(II). Technetium halides exhibit different structure types, such as molecular octahedral complexes, extended chains, layered sheets, and metal clusters arranged in a three-dimensional network. These compounds are produced by combining the metal and halogen or by less direct reactions.
TcCl4 is obtained by chlorination of Tc metal or Tc2O7 Upon heating, TcCl4 gives the corresponding Tc(III) and Tc(II) chlorides.
:TcCl4 → α-TcCl3 + 1/2 Cl2
:TcCl3 → β-TcCl2 + 1/2 Cl2
The structure of TcCl4 is composed of infinite zigzag chains of edge-sharing TcCl6 octahedra. It is isomorphous to transition metal tetrachlorides of zirconium
Zirconium is a chemical element with the symbol Zr and atomic number 40. The name ''zirconium'' is taken from the name of the mineral zircon, the most important source of zirconium. The word is related to Persian '' zargun'' (zircon; ''zar-gun'' ...
, hafnium
Hafnium is a chemical element with the symbol Hf and atomic number 72. A lustrous, silvery gray, tetravalent transition metal, hafnium chemically resembles zirconium and is found in many zirconium minerals. Its existence was predicted by Dmitri M ...
, and platinum
Platinum is a chemical element with the symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal. Its name originates from Spanish , a diminutive of "silver".
Pla ...
.
 Two polymorphs of technetium trichloride exist, α- and β-TcCl3. The α polymorph is also denoted as Tc3Cl9. It adopts a confacial bioctahedral structure. It is prepared by treating the chloro-acetate Tc2(O2CCH3)4Cl2 with HCl. Like Re3Cl9, the structure of the α-polymorph consists of triangles with short M-M distances. β-TcCl3 features octahedral Tc centers, which are organized in pairs, as seen also for molybdenum trichloride. TcBr3 does not adopt the structure of either trichloride phase. Instead it has the structure of molybdenum tribromide, consisting of chains of confacial octahedra with alternating short and long Tc—Tc contacts. TcI3 has the same structure as the high temperature phase of TiI3, featuring chains of confacial octahedra with equal Tc—Tc contacts.
Several anionic technetium halides are known. The binary tetrahalides can be converted to the hexahalides cX6sup>2− (X = F, Cl, Br, I), which adopt
Two polymorphs of technetium trichloride exist, α- and β-TcCl3. The α polymorph is also denoted as Tc3Cl9. It adopts a confacial bioctahedral structure. It is prepared by treating the chloro-acetate Tc2(O2CCH3)4Cl2 with HCl. Like Re3Cl9, the structure of the α-polymorph consists of triangles with short M-M distances. β-TcCl3 features octahedral Tc centers, which are organized in pairs, as seen also for molybdenum trichloride. TcBr3 does not adopt the structure of either trichloride phase. Instead it has the structure of molybdenum tribromide, consisting of chains of confacial octahedra with alternating short and long Tc—Tc contacts. TcI3 has the same structure as the high temperature phase of TiI3, featuring chains of confacial octahedra with equal Tc—Tc contacts.
Several anionic technetium halides are known. The binary tetrahalides can be converted to the hexahalides cX6sup>2− (X = F, Cl, Br, I), which adopt octahedral molecular geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The o ...
. More reduced halides form anionic clusters with Tc–Tc bonds. The situation is similar for the related elements of Mo, W, Re. These clusters have the nuclearity Tc4, Tc6, Tc8, and Tc13. The more stable Tc6 and Tc8 clusters have prism shapes where vertical pairs of Tc atoms are connected by triple bonds and the planar atoms by single bonds. Every technetium atom makes six bonds, and the remaining valence electrons can be saturated by one axial and two bridging ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually ...
halogen atoms such as chlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine i ...
or bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table ( halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simi ...
.
Coordination and organometallic complexes
 Technetium forms a variety of
Technetium forms a variety of coordination complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as '' ligands'' or complexing agents. M ...
es with organic ligands. Many have been well-investigated because of their relevance to nuclear medicine
Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is " radiology done inside out" because it records radiation emi ...
.
Technetium forms a variety of compounds with Tc–C bonds, i.e. organotechnetium complexes. Prominent members of this class are complexes with CO, arene, and cyclopentadienyl ligands. The binary carbonyl Tc2(CO)10 is a white volatile solid. In this molecule, two technetium atoms are bound to each other; each atom is surrounded by octahedra of five carbonyl ligands. The bond length between technetium atoms, 303 pm, is significantly larger than the distance between two atoms in metallic technetium (272 pm). Similar carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containi ...
s are formed by technetium's congeners, manganese and rhenium. Interest in organotechnetium compounds has also been motivated by applications in nuclear medicine
Nuclear medicine or nucleology is a medical specialty involving the application of radioactive substances in the diagnosis and treatment of disease. Nuclear imaging, in a sense, is " radiology done inside out" because it records radiation emi ...
. Technetium also forms aquo-carbonyl complexes, one prominent complex being c(CO)3(H2O)3sup>+, which are unusual compared to other metal carbonyls.
Isotopes
Technetium, withatomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of an atomic nucleus. For ordinary nuclei, this is equal to the proton number (''n''p) or the number of protons found in the nucleus of ever ...
''Z'' = 43, is the lowest-numbered element in the periodic table for which all isotopes are radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consi ...
. The second-lightest exclusively radioactive element, promethium
Promethium is a chemical element with the symbol Pm and atomic number 61. All of its isotopes are radioactive; it is extremely rare, with only about 500–600 grams naturally occurring in Earth's crust at any given time. Promethium is one of onl ...
, has atomic number 61. Atomic nuclei
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron ...
with an odd number of proton
A proton is a stable subatomic particle, symbol , H+, or 1H+ with a positive electric charge of +1 ''e'' elementary charge. Its mass is slightly less than that of a neutron and 1,836 times the mass of an electron (the proton–electron mass ...
s are less stable than those with even numbers, even when the total number of nucleon
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number (nucleon number).
Until the 1960s, nucleons were ...
s (protons + neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
s) is even, and odd numbered elements have fewer stable isotope
Isotopes are two or more types of atoms that have the same atomic number (number of protons in their nuclei) and position in the periodic table (and hence belong to the same chemical element), and that differ in nucleon numbers ( mass num ...
s.
The most stable radioactive isotopes are technetium-97 with a half-life
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ...
of 4.21 million years, technetium-98 with 4.2 million years, and technetium-99 with 211,100 years. Thirty other radioisotopes have been characterized with mass number
The mass number (symbol ''A'', from the German word ''Atomgewicht'' tomic weight, also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approxima ...
s ranging from 85 to 118. Most of these have half-lives that are less than an hour, the exceptions being technetium-93 (2.73 hours), technetium-94 (4.88 hours), technetium-95 (20 hours), and technetium-96 (4.3 days).
The primary decay mode
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is consid ...
for isotopes lighter than technetium-98 (98Tc) is electron capture
Electron capture (K-electron capture, also K-capture, or L-electron capture, L-capture) is a process in which the proton-rich nucleus of an electrically neutral atom absorbs an inner atomic electron, usually from the K or L electron shells. ...
, producing molybdenum
Molybdenum is a chemical element with the symbol Mo and atomic number 42 which is located in period 5 and group 6. The name is from Neo-Latin ''molybdaenum'', which is based on Ancient Greek ', meaning lead, since its ores were confused with lead ...
(''Z'' = 42). For technetium-98 and heavier isotopes, the primary mode is beta emission (the emission of an electron
The electron ( or ) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary particles because they have n ...
or positron
The positron or antielectron is the antiparticle or the antimatter counterpart of the electron. It has an electric charge of +1 '' e'', a spin of 1/2 (the same as the electron), and the same mass as an electron. When a positron collide ...
), producing ruthenium
Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemical ...
(''Z'' = 44), with the exception that technetium-100 can decay both by beta emission and electron capture.
Technetium also has numerous nuclear isomers, which are isotopes with one or more excited nucleons. Technetium-97m (97mTc; "m" stands for metastability
In chemistry and physics, metastability denotes an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball i ...
) is the most stable, with a half-life of 91 days and excitation energy 0.0965 MeV. This is followed by technetium-95m (61 days, 0.03 MeV), and technetium-99m (6.01 hours, 0.142 MeV). Technetium-99m emits only gamma ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically ...
s and decays to technetium-99.
Technetium-99 (99Tc) is a major product of the fission of uranium-235 (235U), making it the most common and most readily available isotope of technetium. One gram of technetium-99 produces 6.2×108 disintegrations per second (in other words, the specific activity of 99Tc is 0.62 G Bq/g).
Occurrence and production
Technetium occurs naturally in the Earth's crust in minute concentrations of about 0.003 parts per trillion. Technetium is so rare because thehalf-lives
Half-life (symbol ) is the time required for a quantity (of substance) to reduce to half of its initial value. The term is commonly used in nuclear physics to describe how quickly unstable atoms undergo radioactive decay or how long stable ato ...
of 97Tc and 98Tc are only 4.2 million years. More than a thousand of such periods have passed since the formation of the Earth
Earth is the third planet from the Sun and the only astronomical object known to harbor life. While large volumes of water can be found throughout the Solar System, only Earth sustains liquid surface water. About 71% of Earth's sur ...
, so the probability of survival of even one atom of primordial
Primordial may refer to:
* Primordial era, an era after the Big Bang. See Chronology of the universe
* Primordial sea (a.k.a. primordial ocean, ooze or soup). See Abiogenesis
* Primordial nuclide, nuclides, a few radioactive, that formed before t ...
technetium is effectively zero. However, small amounts exist as spontaneous fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
s in uranium ores. A kilogram of uranium contains an estimated 1 nanogram
To help compare different orders of magnitude, the following lists describe various mass levels between 10−59 kg and 1052 kg. The least massive thing listed here is a graviton, and the most massive thing is the observable universe ...
(10−9 g) equivalent to ten trillion atoms of technetium. Some red giant
A red giant is a luminous giant star of low or intermediate mass (roughly 0.3–8 solar masses ()) in a late phase of stellar evolution. The outer atmosphere is inflated and tenuous, making the radius large and the surface temperature around o ...
stars with the spectral types S-, M-, and N contain a spectral absorption line indicating the presence of technetium. These red giants are known informally as technetium stars.
Fission waste product
In contrast to the rare natural occurrence, bulk quantities of technetium-99 are produced each year from spent nuclear fuel rods, which contain various fission products. The fission of a gram ofuranium-235
Uranium-235 (235U or U-235) is an isotope of uranium making up about 0.72% of natural uranium. Unlike the predominant isotope uranium-238, it is fissile, i.e., it can sustain a nuclear chain reaction. It is the only fissile isotope that exi ...
in nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
s yields 27 mg of technetium-99, giving technetium a fission product yield
Nuclear fission splits a heavy nucleus such as uranium or plutonium into two lighter nuclei, which are called fission products. Yield refers to the fraction of a fission product produced per fission.
Yield can be broken down by:
# Individual ...
of 6.1%. Other fissile
In nuclear engineering, fissile material is material capable of sustaining a nuclear fission chain reaction. By definition, fissile material can sustain a chain reaction with neutrons of thermal energy. The predominant neutron energy may be t ...
isotopes produce similar yields of technetium, such as 4.9% from uranium-233
Uranium-233 (233U or U-233) is a fissile isotope of uranium that is bred from thorium-232 as part of the thorium fuel cycle. Uranium-233 was investigated for use in nuclear weapons and as a reactor fuel. It has been used successfully in exp ...
and 6.21% from plutonium-239
Plutonium-239 (239Pu or Pu-239) is an isotope of plutonium. Plutonium-239 is the primary fissile isotope used for the production of nuclear weapons, although uranium-235 is also used for that purpose. Plutonium-239 is also one of the three mai ...
. An estimated 49,000 T Bq (78 metric tons
The tonne ( or ; symbol: t) is a unit of mass equal to 1000 kilograms. It is a non-SI unit accepted for use with SI. It is also referred to as a metric ton to distinguish it from the non-metric units of the short ton ( United State ...
) of technetium was produced in nuclear reactors between 1983 and 1994, by far the dominant source of terrestrial technetium. Only a fraction of the production is used commercially.
Technetium-99 is produced by the nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radio ...
of both uranium-235 and plutonium-239. It is therefore present in radioactive waste
Radioactive waste is a type of hazardous waste that contains radioactive material. Radioactive waste is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, rare-earth mining, and nuclear weapon ...
and in the nuclear fallout
Nuclear fallout is the residual radioactive material propelled into the upper atmosphere following a nuclear blast, so called because it "falls out" of the sky after the explosion and the shock wave has passed. It commonly refers to the radioac ...
of fission bomb
A nuclear weapon is an explosive device that derives its destructive force from nuclear reactions, either fission (fission bomb) or a combination of fission and fusion reactions ( thermonuclear bomb), producing a nuclear explosion. Both bomb ...
explosions. Its decay, measured in becquerel
The becquerel (; symbol: Bq) is the unit of radioactivity in the International System of Units (SI). One becquerel is defined as the activity of a quantity of radioactive material in which one nucleus decays per second. For applications relatin ...
s per amount of spent fuel, is the dominant contributor to nuclear waste radioactivity after about 104 to 106 years after the creation of the nuclear waste. From 1945 to 1994, an estimated 160 T Bq (about 250 kg) of technetium-99 was released into the environment during atmospheric nuclear tests. The amount of technetium-99 from nuclear reactors released into the environment up to 1986 is on the order of 1000 TBq (about 1600 kg), primarily by nuclear fuel reprocessing; most of this was discharged into the sea. Reprocessing methods have reduced emissions since then, but as of 2005 the primary release of technetium-99 into the environment is by the Sellafield
Sellafield is a large multi-function nuclear site close to Seascale on the coast of Cumbria, England. As of August 2022, primary activities are nuclear waste processing and storage and nuclear decommissioning. Former activities included nuc ...
plant, which released an estimated 550 TBq (about 900 kg) from 1995 to 1999 into the Irish Sea
The Irish Sea or , gv, Y Keayn Yernagh, sco, Erse Sie, gd, Muir Èireann , Ulster-Scots: ''Airish Sea'', cy, Môr Iwerddon . is an extensive body of water that separates the islands of Ireland and Great Britain. It is linked to the C ...
. From 2000 onwards the amount has been limited by regulation to 90 TBq (about 140 kg) per year. Discharge of technetium into the sea resulted in contamination of some seafood with minuscule quantities of this element. For example, European lobster and fish from west Cumbria
Cumbria ( ) is a ceremonial and non-metropolitan county in North West England, bordering Scotland. The county and Cumbria County Council, its local government, came into existence in 1974 after the passage of the Local Government Act 1972. ...
contain about 1 Bq/kg of technetium.
Fission product for commercial use
The metastable isotope technetium-99m is continuously produced as afission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
from the fission of uranium or plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exh ...
in nuclear reactor
A nuclear reactor is a device used to initiate and control a fission nuclear chain reaction or nuclear fusion reactions. Nuclear reactors are used at nuclear power plants for electricity generation and in nuclear marine propulsion. Heat fr ...
s:
:actinide
The actinide () or actinoid () series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. The actinide series derives its name from the first element in the series, actinium. The info ...
s in conventional nuclear reprocessing
Nuclear reprocessing is the chemical separation of fission products and actinides from spent nuclear fuel. Originally, reprocessing was used solely to extract plutonium for producing nuclear weapons. With commercialization of nuclear power, th ...
. The liquid left after plutonium–uranium extraction (PUREX
PUREX (plutonium uranium reduction extraction) is a chemical method used to purify fuel for nuclear reactors or nuclear weapons. PUREX is the '' de facto'' standard aqueous nuclear reprocessing method for the recovery of uranium and pluto ...
) contains a high concentration of technetium as but almost all of this is technetium-99, not technetium-99m.
The vast majority of the technetium-99m used in medical work is produced by irradiating dedicated highly enriched uranium
Enriched uranium is a type of uranium in which the percent composition of uranium-235 (written 235U) has been increased through the process of isotope separation. Naturally occurring uranium is composed of three major isotopes: uranium-238 (238U ...
targets in a reactor, extracting molybdenum-99 from the targets in reprocessing facilities, and recovering at the diagnostic center the technetium-99m produced upon decay of molybdenum-99. Molybdenum-99 in the form of molybdate is adsorbed onto acid alumina () in a shielded column chromatograph inside a technetium-99m generator ("technetium cow", also occasionally called a "molybdenum cow"). Molybdenum-99 has a half-life of 67 hours, so short-lived technetium-99m (half-life: 6 hours), which results from its decay, is being constantly produced. The soluble pertechnetate can then be chemically extracted by elution using a saline solution
Saline (also known as saline solution) is a mixture of sodium chloride (salt) and water. It has a number of uses in medicine including cleaning wounds, removal and storage of contact lenses, and help with dry eyes. By injection into a vein ...
. A drawback of this process is that it requires targets containing uranium-235, which are subject to the security precautions of fissile materials.
 Almost two-thirds of the world's supply comes from two reactors; the National Research Universal Reactor at
Almost two-thirds of the world's supply comes from two reactors; the National Research Universal Reactor at Chalk River Laboratories
Chalk River Laboratories (french: Laboratoires de Chalk River; also known as CRL, Chalk River Labs and formerly Chalk River Nuclear Laboratories, CRNL) is a Canadian nuclear research facility in Deep River, about north-west of Ottawa.
CRL is ...
in Ontario, Canada, and the High Flux Reactor at Nuclear Research and Consultancy Group Nuclear Research and Consultancy Group (NRG) is a Dutch institute that performs nuclear research for the government and private companies. It is the most important producer of radionuclides, such as molybdenum-99, lutetium-177 and iridium-192, in ...
in Petten, Netherlands. All major reactors that produce technetium-99m were built in the 1960s and are close to the end of life End-of-life may refer to:
* End-of-life (product), a term used with respect to terminating the sale or support of goods and services
* End-of-life care
End-of-life care (EoLC) refers to health care provided in the time leading up to a person's dea ...
. The two new Canadian Multipurpose Applied Physics Lattice Experiment reactors planned and built to produce 200% of the demand of technetium-99m relieved all other producers from building their own reactors. With the cancellation of the already tested reactors in 2008, the future supply of technetium-99m became problematic.
Waste disposal
The long half-life of technetium-99 and its potential to formanionic
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conven ...
species creates a major concern for long-term disposal of radioactive waste Radioactive waste disposal may refer to:
*High-level radioactive waste management
* Low-level waste disposal
* Ocean disposal of radioactive waste
** Ocean floor disposal
* Deep borehole disposal
*Deep geological repository
A deep geological repo ...
. Many of the processes designed to remove fission products in reprocessing plants aim at cationic species such as caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
(e.g., caesium-137
Caesium-137 (), cesium-137 (US), or radiocaesium, is a radioactive isotope of caesium that is formed as one of the more common fission products by the nuclear fission of uranium-235 and other fissionable isotopes in nuclear reactors and nucle ...
) and strontium
Strontium is the chemical element with the symbol Sr and atomic number 38. An alkaline earth metal, strontium is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is e ...
(e.g., strontium-90
Strontium-90 () is a radioactive isotope of strontium produced by nuclear fission, with a half-life of 28.8 years. It undergoes β− decay into yttrium-90, with a decay energy of 0.546 MeV. Strontium-90 has applications in medicine and ...
). Hence the pertechnetate escapes through those processes. Current disposal options favor burial
Burial, also known as interment or inhumation, is a method of final disposition whereby a dead body is placed into the ground, sometimes with objects. This is usually accomplished by excavating a pit or trench, placing the deceased and objec ...
in continental, geologically stable rock. The primary danger with such practice is the likelihood that the waste will contact water, which could leach radioactive contamination into the environment. The anionic pertechnetate and iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine de ...
tend not to adsorb into the surfaces of minerals, and are likely to be washed away. By comparison plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exh ...
, uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weak ...
, and caesium
Caesium (IUPAC spelling) (or cesium in American English) is a chemical element with the symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that a ...
tend to bind to soil particles. Technetium could be immobilized by some environments, such as microbial activity in lake bottom sediments, and the environmental chemistry
Environmental chemistry is the scientific study of the chemical and biochemical phenomena that occur in natural places. It should not be confused with green chemistry, which seeks to reduce potential pollution at its source. It can be defined as ...
of technetium is an area of active research.
An alternative disposal method, transmutation, has been demonstrated at CERN
The European Organization for Nuclear Research, known as CERN (; ; ), is an intergovernmental organization that operates the largest particle physics laboratory in the world. Established in 1954, it is based in a northwestern suburb of Gen ...
for technetium-99. In this process, the technetium (technetium-99 as a metal target) is bombarded with neutron
The neutron is a subatomic particle, symbol or , which has a neutral (not positive or negative) charge, and a mass slightly greater than that of a proton. Protons and neutrons constitute the atomic nucleus, nuclei of atoms. Since protons and ...
s to form the short-lived technetium-100 (half-life = 16 seconds) which decays by beta decay to stable ruthenium
Ruthenium is a chemical element with the symbol Ru and atomic number 44. It is a rare transition metal belonging to the platinum group of the periodic table. Like the other metals of the platinum group, ruthenium is inert to most other chemical ...
-100. If recovery of usable ruthenium is a goal, an extremely pure technetium target is needed; if small traces of the minor actinide
The minor actinides are the actinide elements in used nuclear fuel other than uranium and plutonium, which are termed the major actinides. The minor actinides include neptunium (element 93), americium (element 95), curium (element 96), berkeliu ...
s such as americium
Americium is a synthetic radioactive chemical element with the symbol Am and atomic number 95. It is a transuranic member of the actinide series, in the periodic table located under the lanthanide element europium, and thus by analogy was n ...
and curium
Curium is a transuranic, radioactive chemical element with the symbol Cm and atomic number 96. This actinide element was named after eminent scientists Marie and Pierre Curie, both known for their research on radioactivity. Curium was first in ...
are present in the target, they are likely to undergo fission and form more fission product
Nuclear fission products are the atomic fragments left after a large atomic nucleus undergoes nuclear fission. Typically, a large nucleus like that of uranium fissions by splitting into two smaller nuclei, along with a few neutrons, the release ...
s which increase the radioactivity of the irradiated target. The formation of ruthenium-106 (half-life 374 days) from the 'fresh fission' is likely to increase the activity of the final ruthenium metal, which will then require a longer cooling time after irradiation before the ruthenium can be used.
The actual separation of technetium-99 from spent nuclear fuel is a long process. During fuel reprocessing, it comes out as a component of the highly radioactive waste liquid. After sitting for several years, the radioactivity reduces to a level where extraction of the long-lived isotopes, including technetium-99, becomes feasible. A series of chemical processes yields technetium-99 metal of high purity.
Neutron activation
Molybdenum-99
Molybdenum (42Mo) has 33 known isotopes, ranging in atomic mass from 83 to 115, as well as four metastable nuclear isomers. Seven isotopes occur naturally, with atomic masses of 92, 94, 95, 96, 97, 98, and 100. All unstable isotopes of molybdenum ...
, which decays to form technetium-99m, can be formed by the neutron activation
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus decays immediately by emit ...
of molybdenum-98. When needed, other technetium isotopes are not produced in significant quantities by fission, but are manufactured by neutron irradiation of parent isotopes (for example, technetium-97 can be made by neutron irradiation of ruthenium-96).
Particle accelerators
The feasibility of technetium-99m production with the 22-MeV-proton bombardment of a molybdenum-100 target in medical cyclotrons following the reaction 100Mo(p,2n)99mTc was demonstrated in 1971. The recent shortages of medical technetium-99m reignited the interest in its production by proton bombardment of isotopically enriched (>99.5%) molybdenum-100 targets. Other techniques are being investigated for obtaining molybdenum-99 from molybdenum-100 via (n,2n) or (γ,n) reactions in particle accelerators.Applications
Nuclear medicine and biology

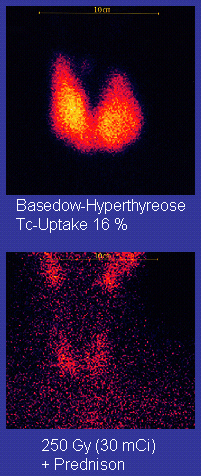
Technetium-99m
Technetium-99m (99mTc) is a metastable nuclear isomer of technetium-99 (itself an isotope of technetium), symbolized as 99mTc, that is used in tens of millions of medical diagnostic procedures annually, making it the most commonly used medica ...
("m" indicates that this is a metastable nuclear isomer) is used in radioactive isotope medical tests
A medical test is a medical procedure performed to detect, diagnose, or monitor diseases, disease processes, susceptibility, or to determine a course of treatment. Medical tests such as, physical and visual exams, diagnostic imaging, genetic t ...
. For example, Technetium-99m is a radioactive tracer
A radioactive tracer, radiotracer, or radioactive label is a chemical compound in which one or more atoms have been replaced by a radionuclide so by virtue of its radioactive decay it can be used to explore the mechanism of chemical reactions by ...
that medical imaging equipment tracks in the human body. It is well suited to the role because it emits readily detectable 140 keV gamma ray
A gamma ray, also known as gamma radiation (symbol γ or \gamma), is a penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei. It consists of the shortest wavelength electromagnetic waves, typically ...
s, and its half-life is 6.01 hours (meaning that about 94% of it decays to technetium-99 in 24 hours). The chemistry of technetium allows it to be bound to a variety of biochemical compounds, each of which determines how it is metabolized and deposited in the body, and this single isotope can be used for a multitude of diagnostic tests. More than 50 common radiopharmaceuticals are based on technetium-99m for imaging and functional studies of the brain
A brain is an organ (biology), organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. It is located in the head, usually close to the sensory organs for senses such as Visual perception, vision. I ...
, heart muscle, thyroid
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans it is in the neck and consists of two connected lobes. The lower two thirds of the lobes are connected by a thin band of tissue called the thyroid isthmus. The ...
, lungs
The lungs are the primary organs of the respiratory system in humans and most other animals, including some snails and a small number of fish. In mammals and most other vertebrates, two lungs are located near the backbone on either si ...
, liver
The liver is a major organ only found in vertebrates which performs many essential biological functions such as detoxification of the organism, and the synthesis of proteins and biochemicals necessary for digestion and growth. In humans, it i ...
, gall bladder
In vertebrates, the gallbladder, also known as the cholecyst, is a small hollow organ where bile is stored and concentrated before it is released into the small intestine. In humans, the pear-shaped gallbladder lies beneath the liver, although ...
, kidney
The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; blo ...
s, skeleton
A skeleton is the structural frame that supports the body of an animal. There are several types of skeletons, including the exoskeleton, which is the stable outer shell of an organism, the endoskeleton, which forms the support structure inside ...
, blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood in the cir ...
, and tumor
A neoplasm () is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists ...
s.
The longer-lived isotope, technetium-95m with a half-life of 61 days, is used as a radioactive tracer
A radioactive tracer, radiotracer, or radioactive label is a chemical compound in which one or more atoms have been replaced by a radionuclide so by virtue of its radioactive decay it can be used to explore the mechanism of chemical reactions by ...
to study the movement of technetium in the environment and in plant and animal systems.
Industrial and chemical
Technetium-99 decays almost entirely by beta decay, emitting beta particles with consistent low energies and no accompanying gamma rays. Moreover, its long half-life means that this emission decreases very slowly with time. It can also be extracted to a high chemical and isotopic purity from radioactive waste. For these reasons, it is aNational Institute of Standards and Technology
The National Institute of Standards and Technology (NIST) is an agency of the United States Department of Commerce whose mission is to promote American innovation and industrial competitiveness. NIST's activities are organized into physical s ...
(NIST) standard beta emitter, and is used for equipment calibration. Technetium-99 has also been proposed for optoelectronic devices and nanoscale
The nanoscopic scale (or nanoscale) usually refers to structures with a length scale applicable to nanotechnology, usually cited as 1–100 nanometers (nm). A nanometer is a billionth of a meter. The nanoscopic scale is (roughly speaking) a lo ...
nuclear batteries.
Like rhenium
Rhenium is a chemical element with the symbol Re and atomic number 75. It is a silvery-gray, heavy, third-row transition metal in group 7 of the periodic table. With an estimated average concentration of 1 part per billion (ppb), rhenium is one ...
and palladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself ...
, technetium can serve as a catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
. In processes such as the dehydrogenation
In chemistry, dehydrogenation is a chemical reaction that involves the removal of hydrogen, usually from an organic molecule. It is the reverse of hydrogenation. Dehydrogenation is important, both as a useful reaction and a serious problem. A ...
of isopropyl alcohol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group ( chemical formula ) it is the s ...
, it is a far more effective catalyst than either rhenium or palladium. However, its radioactivity is a major problem in safe catalytic applications.
When steel is immersed in water, adding a small concentration (55 ppm) of potassium pertechnetate(VII) to the water protects the steel
Steel is an alloy made up of iron with added carbon to improve its strength and fracture resistance compared to other forms of iron. Many other elements may be present or added. Stainless steels that are corrosion- and oxidation-resistan ...
from corrosion, even if the temperature is raised to . For this reason, pertechnetate has been used as an anodic corrosion
Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials (usually a metal) by chemical or electrochemical reaction with their environment. Corrosion engi ...
inhibitor for steel, although technetium's radioactivity poses problems that limit this application to self-contained systems. While (for example) can also inhibit corrosion, it requires a concentration ten times as high. In one experiment, a specimen of carbon steel was kept in an aqueous solution of pertechnetate for 20 years and was still uncorroded. The mechanism by which pertechnetate prevents corrosion is not well understood, but seems to involve the reversible formation of a thin surface layer ( passivation). One theory holds that the pertechnetate reacts with the steel surface to form a layer of technetium dioxide
An oxide () is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. "Oxide" itself is the dianion of oxygen, an O2– (molecular) ion. with oxygen in the oxidation state of −2. Most of ...
which prevents further corrosion; the same effect explains how iron powder can be used to remove pertechnetate from water. The effect disappears rapidly if the concentration of pertechnetate falls below the minimum concentration or if too high a concentration of other ions is added.
As noted, the radioactive nature of technetium (3 MBq/L at the concentrations required) makes this corrosion protection impractical in almost all situations. Nevertheless, corrosion protection by pertechnetate ions was proposed (but never adopted) for use in boiling water reactors.
Precautions
Technetium plays no natural biological role and is not normally found in the human body. Technetium is produced in quantity by nuclear fission, and spreads more readily than many radionuclides. It appears to have low chemical toxicity. For example, no significant change in blood formula, body and organ weights, and food consumption could be detected for rats which ingested up to 15 µg of technetium-99 per gram of food for several weeks. In the body, technetium quickly gets converted to the stable ion, which is highly water-soluble and quickly excreted. The radiological toxicity of technetium (per unit of mass) is a function of compound, type of radiation for the isotope in question, and the isotope's half-life. All isotopes of technetium must be handled carefully. The most common isotope, technetium-99, is a weak beta emitter; such radiation is stopped by the walls of laboratory glassware. The primary hazard when working with technetium is inhalation of dust; suchradioactive contamination
Radioactive contamination, also called radiological pollution, is the deposition of, or presence of radioactive substances on surfaces or within solids, liquids, or gases (including the human body), where their presence is unintended or undesirab ...
in the lungs can pose a significant cancer risk. For most work, careful handling in a fume hood is sufficient, and a glove box is not needed.
Notes
References
Bibliography
* * * *Further reading
* * * *EnvironmentalChemistry.com – Technetium
!--per the guidelines a
Wikipedia's WikiProject Elements
(all viewed 1 December 2002)-->
Nudat 2
nuclide chart from the National Nuclear Data Center, Brookhaven National Laboratory
External links
at '' The Periodic Table of Videos'' (University of Nottingham) {{Authority control Chemical elements Transition metals Synthetic elements Radiopharmaceuticals Chemical elements predicted by Dmitri Mendeleev Chemical elements with hexagonal close-packed structure