|
Pyrrolidine
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like". In addition to pyrrolidine itself, many substituted pyrrolidines are known. Production and synthesis Industrial production Pyrrolidine is prepared industrially by the reaction of 1,4-butanediol and ammonia at a temperature of 165–200 °C and a pressure of 17–21 MPa in the presence of a cobalt- and nickel oxide catalyst, which is supported on alumina. : The reaction is carried out in the liquid phase in a continuous tube- or tube bundle reactor, which is operated in the cycle gas method. The catalyst is arranged as a fixed-bed and the conversion is carried out in the downflow mode. The product is obtained after ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrrole
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4 H4 NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., ''N''-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme. Pyrroles are components of more complex macrocycles, including the porphyrinogens and products derived therefrom, including porphyrins of heme, the chlorins, bacteriochlorins, and chlorophylls. Properties Pyrrole is a colorless volatile liquid that darkens readily upon exposure to air, and is usually purified by distillation immediately before use. Pyrrole has a nutty odor. Pyrrole is a 5-membered aromatic heterocycle, like furan and thiophene. Unlike furan and thiophene, it has a dipole in which the positive end lies on the side of the heteroatom, with a dipole moment of 1.58 D. In CDCl3, it ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
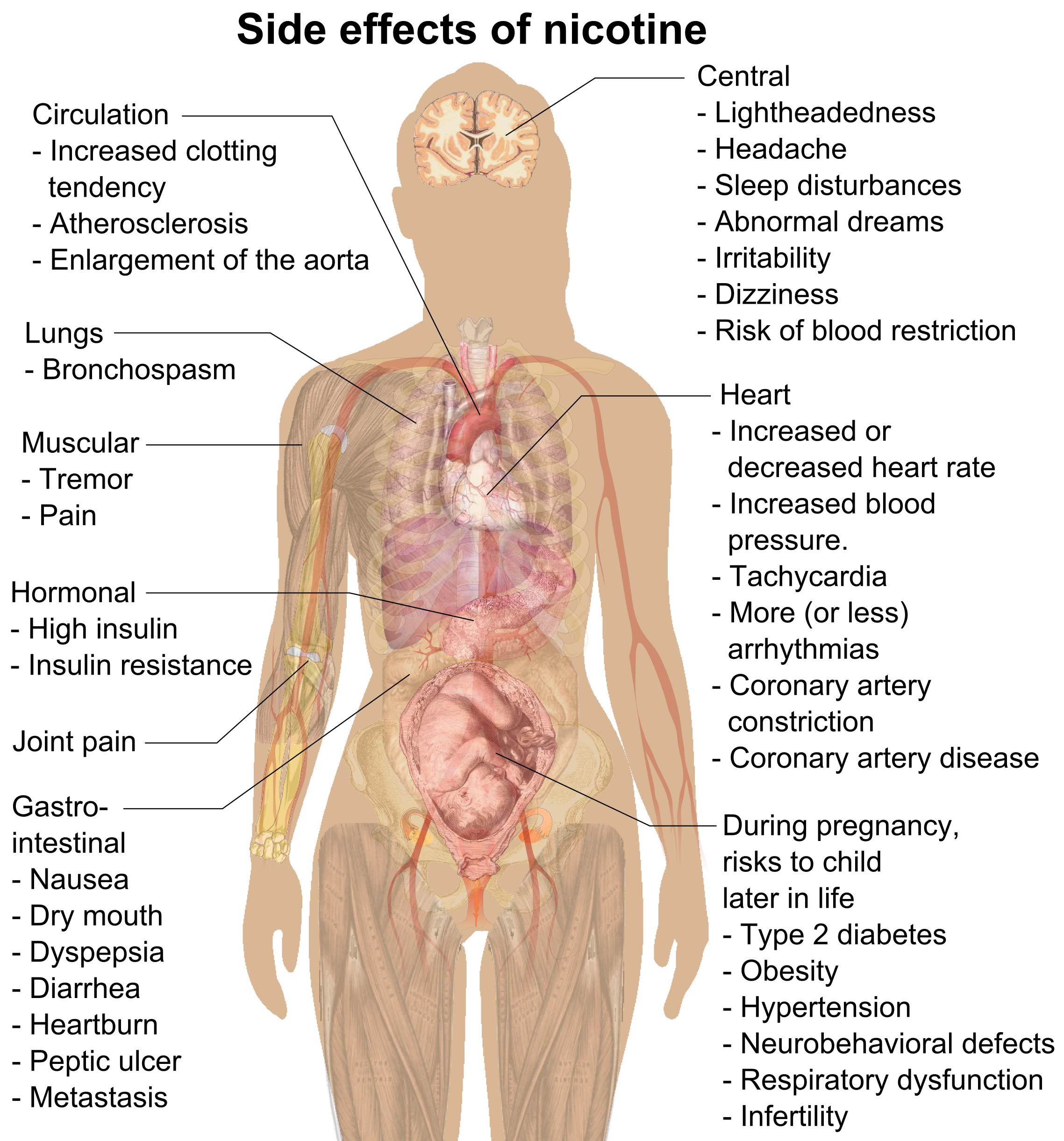
Nicotine
Nicotine is a naturally produced alkaloid in the nightshade family of plants (most predominantly in tobacco and '' Duboisia hopwoodii'') and is widely used recreationally as a stimulant and anxiolytic. As a pharmaceutical drug, it is used for smoking cessation to relieve withdrawal symptoms. Nicotine acts as a receptor agonist at most nicotinic acetylcholine receptors (nAChRs), except at two nicotinic receptor subunits ( nAChRα9 and nAChRα10) where it acts as a receptor antagonist. Nicotine constitutes approximately 0.6–3.0% of the dry weight of tobacco. Nicotine is also present at ppb-concentrations in edible plants in the family Solanaceae, including potatoes, tomatoes, and eggplants, though sources disagree on whether this has any biological significance to human consumers. It functions as an antiherbivore toxin; consequently, nicotine was widely used as an insecticide in the past, and neonicotinoids (structurally similar to nicotine), such as imidaclopri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
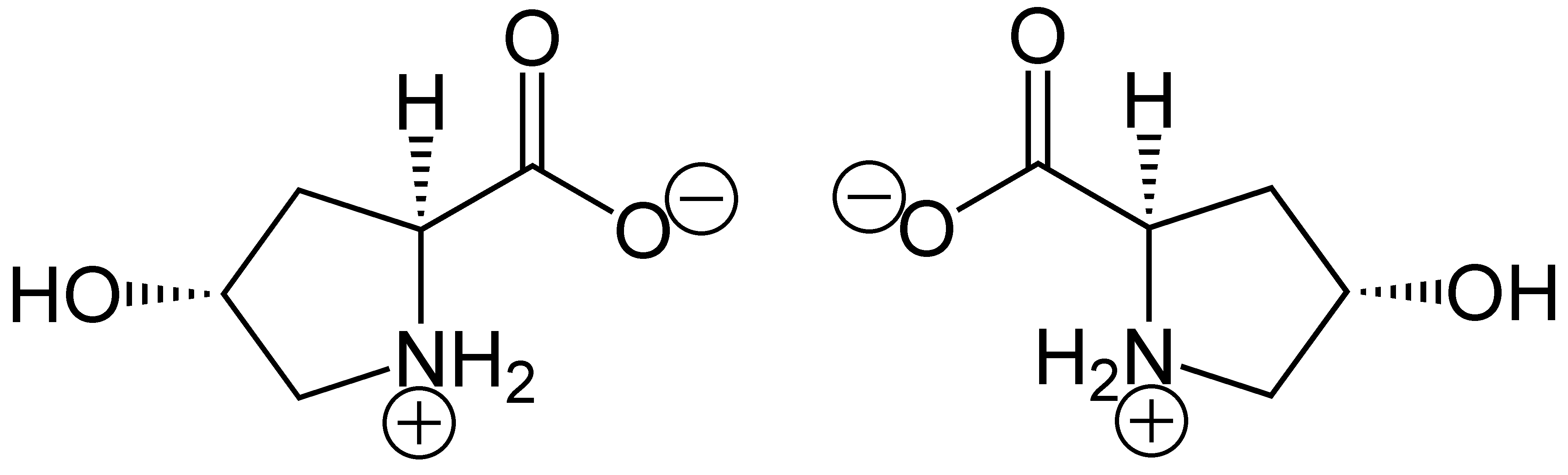
Proline
Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG). Proline is the only proteinogenic secondary amino acid which is a secondary amine, as the nitrogen atom is attached both to the α-carbon and to a chain of three carbons that together form a five-membered ring. History and etymology Proline was first isolated in 1900 by Richard Willstätter who obtained t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotine
Nicotine is a naturally produced alkaloid in the nightshade family of plants (most predominantly in tobacco and '' Duboisia hopwoodii'') and is widely used recreationally as a stimulant and anxiolytic. As a pharmaceutical drug, it is used for smoking cessation to relieve withdrawal symptoms. Nicotine acts as a receptor agonist at most nicotinic acetylcholine receptors (nAChRs), except at two nicotinic receptor subunits ( nAChRα9 and nAChRα10) where it acts as a receptor antagonist. Nicotine constitutes approximately 0.6–3.0% of the dry weight of tobacco. Nicotine is also present at ppb-concentrations in edible plants in the family Solanaceae, including potatoes, tomatoes, and eggplants, though sources disagree on whether this has any biological significance to human consumers. It functions as an antiherbivore toxin; consequently, nicotine was widely used as an insecticide in the past, and neonicotinoids (structurally similar to nicotine), such as imidaclopri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaloid
Alkaloids are a class of basic BASIC (Beginners' All-purpose Symbolic Instruction Code) is a family of general-purpose, high-level programming languages designed for ease of use. The original version was created by John G. Kemeny and Thomas E. Kurtz at Dartmouth College ..., natural product, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.Chemical Encyclopedia: alkaloids xumuk.ru Alkaloids are produced by a large variety of organisms includi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyproline
(2''S'',4''R'')-4-Hydroxyproline, or L-hydroxyproline ( C5 H9 O3 N), is an amino acid, abbreviated as Hyp or O, ''e.g.'', in Protein Data Bank. Structure and discovery In 1902, Hermann Emil Fischer isolated hydroxyproline from hydrolyzed gelatin. In 1905, Hermann Leuchs synthesized a racemic mixture of 4-hydroxyproline. Hydroxyproline differs from proline by the presence of a hydroxyl (OH) group attached to the gamma carbon atom. Production and function Hydroxyproline is produced by hydroxylation of the amino acid proline by the enzyme prolyl hydroxylase following protein synthesis (as a post-translational modification). The enzyme catalyzed reaction takes place in the lumen of the endoplasmic reticulum. Although it is not directly incorporated into proteins, hydroxyproline comprises roughly 4% of all amino acids found in animal tissue, an amount greater than seven other amino acids that are translationally incorporated. Animals Collagen Hydroxyproline is a major co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piracetam
Piracetam is a drug marketed as a treatment for myoclonus. It is also used as a cognitive enhancer to improve memory, attention, and learning. Evidence to support its use is unclear, with some studies showing modest benefits in specific populations and others showing minimal or no benefit. Piracetam is sold as a medication in many European countries. Sale of piracetam is not illegal in the United States, although it is not regulated nor approved by the FDA so it is legally sold for research use only. Piracetam is in the racetams group, with chemical name ''2-oxo-1-pyrrolidine acetamide''. It is a derivative of the neurotransmitter GABA and shares the same 2-oxo-pyrrolidone base structure with pyroglutamic acid. Piracetam is a cyclic derivative of GABA (gamma-aminobutyric acid). Related drugs include the anticonvulsants levetiracetam and brivaracetam, and the putative nootropics aniracetam and phenylpiracetam. Efficacy Dementia A 2001 Cochrane review concluded that there ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hygrine
Hygrine is a pyrrolidine alkaloid, found mainly in coca leaves (0.2%). It was first isolated by Carl Liebermann in 1889 (along with a related compound cuscohygrine) as an alkaloid accompanying cocaine Cocaine (from , from , ultimately from Quechua: ''kúka'') is a central nervous system (CNS) stimulant mainly used recreationally for its euphoric effects. It is primarily obtained from the leaves of two Coca species native to South Am ... in coca. Hygrine is extracted as a thick yellow oil, having a pungent taste and odor. See also * Coca alkaloids * Pseudotropine * Troparil References * * * {{cite web, title=USDA, ARS, National Genetic Resources Program. Phytochemical and Ethnobotanical Databases. National Germplasm Resources Laboratory, Beltsville, Maryland. , url=http://sun.ars-grin.gov:8080/npgspub/xsql/duke/chemdisp.xsql?chemical=HYGRINE , archive-url=https://archive.today/20121211210941/http://sun.ars-grin.gov:8080/npgspub/xsql/duke/chemdisp.xsql?chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cascade Reaction
A cascade reaction, also known as a domino reaction or tandem reaction, is a chemical process that comprises at least two consecutive reactions such that each subsequent reaction occurs only in virtue of the chemical functionality formed in the previous step.Tietze, L. F.; Beifuss, U. ''Angew. Chem. Int. Ed.'' 1993, ''32'', 131–163. In cascade reactions, isolation of intermediates is not required, as each reaction composing the sequence occurs spontaneously. In the strictest definition of the term, the reaction conditions do not change among the consecutive steps of a cascade and no new reagents are added after the initial step.Padwa, A.; Bur, S. K. ''Tetrahedron'' 2007, ''63'', 5341–5378. By contrast, one-pot procedures similarly allow at least two reactions to be carried out consecutively without any isolation of intermediates, but do not preclude the addition of new reagents or the change of conditions after the first reaction. Thus, any cascade reaction is also a one-pot p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although hundreds of amino acids exist in nature, by far the most important are the alpha-amino acids, which comprise proteins. Only 22 alpha amino acids appear in the genetic code. Amino acids can be classified according to the locations of the core structural functional groups, as Alpha and beta carbon, alpha- , beta- , gamma- or delta- amino acids; other categories relate to Chemical polarity, polarity, ionization, and side chain group type (aliphatic, Open-chain compound, acyclic, aromatic, containing hydroxyl or sulfur, etc.). In the form of proteins, amino acid ''residues'' form the second-largest component ( water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling li ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniracetam
Aniracetam (brand names Draganon, Sarpul, Ampamet, Memodrin, Referan), also known as ''N''-anisoyl-2-pyrrolidinone, is a racetam which is sold in Europe as a prescription drug. It is not approved by the Food and Drug Administration for use in the United States as a prescription medication or dietary supplement. Despite the FDA's lack of approval, the drug is readily available over-the-counter in the US as a dietary supplement. Pharmacology Aniracetam has been shown to positively modulate the AMPA receptor. When ingested orally aniracetam is quickly broken down via first pass hepatic metabolism. The primary metabolites of aniracetam are N-anisoyl-GABA, ''N''-anisoyl-GABA, (70–80%), 2-Pyrrolidinone and p-Anisic acid, ''p''-anisic acid (20–30%). Plasma concentrations are generally in the 5–15 ''μ''g/L range for aniracetam and 5–15 mg/L range for ''N''-anisoyl-GABA, a pharmacologically-active metabolite, during the first few hours after oral administration of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |