|
Psychrometric Constant
The psychrometric constant \gamma relates the partial pressure of water in air to the air temperature. This lets one interpolate actual vapor pressure from paired dry and wet thermometer bulb temperature readings. :: \gamma =\frac : \gamma = psychrometric constant Pa °C−1 : P = atmospheric pressure Pa : \lambda_v = latent heat of water vaporization, 2.45 J kg−1 : c_p = specific heat of air at constant pressure, J kg−1 °C−1 : MW_ = ratio molecular weight of water vapor/dry air = 0.622. Both \lambda_v and MW_ are constants. Since atmospheric pressure, P, depends upon altitude, so does \gamma. At higher altitude water evaporates and boils at lower temperature. Although \left( c_p \right)_ is constant, varied air composition results in varied \left( c_p \right)_ . Thus on average, at a given location or altitude, the psychrometric constant is approximately constant. Still, it is worth remembering that weather impacts both atmospheric pressure a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
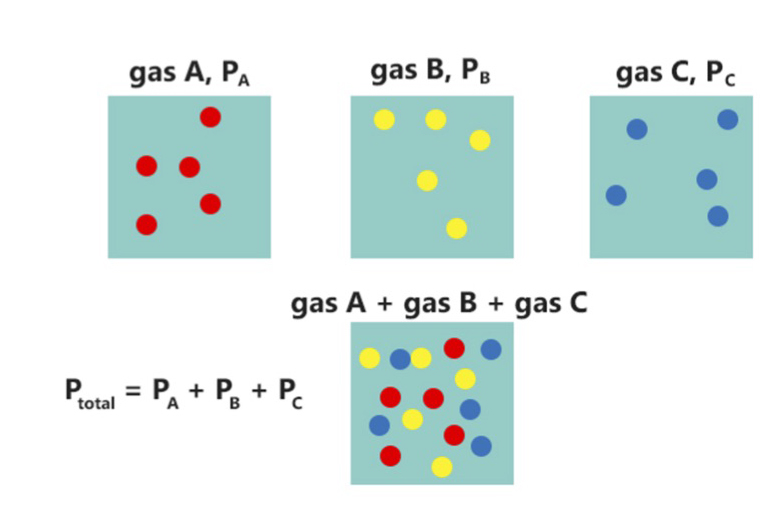
Partial Pressure
In a mixture of gases, each constituent gas has a partial pressure which is the notional pressure of that constituent gas as if it alone occupied the entire volume of the original mixture at the same temperature. The total pressure of an ideal gas mixture is the sum of the partial pressures of the gases in the mixture (Dalton's Law). The partial pressure of a gas is a measure of thermodynamic activity of the gas's molecules. Gases dissolve, diffuse, and react according to their partial pressures but not according to their concentrations in gas mixtures or liquids. This general property of gases is also true in chemical reactions of gases in biology. For example, the necessary amount of oxygen for human respiration, and the amount that is toxic, is set by the partial pressure of oxygen alone. This is true across a very wide range of different concentrations of oxygen present in various inhaled breathing gases or dissolved in blood; consequently, mixture ratios, like that of breathab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interpolate
In the mathematical field of numerical analysis, interpolation is a type of estimation, a method of constructing (finding) new data points based on the range of a discrete set of known data points. In engineering and science, one often has a number of data points, obtained by sampling or experimentation, which represent the values of a function for a limited number of values of the independent variable. It is often required to interpolate; that is, estimate the value of that function for an intermediate value of the independent variable. A closely related problem is the approximation of a complicated function by a simple function. Suppose the formula for some given function is known, but too complicated to evaluate efficiently. A few data points from the original function can be interpolated to produce a simpler function which is still fairly close to the original. The resulting gain in simplicity may outweigh the loss from interpolation error and give better performance in c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermometer
A thermometer is a device that temperature measurement, measures temperature or a temperature gradient (the degree of hotness or coldness of an object). A thermometer has two important elements: (1) a temperature sensor (e.g. the bulb of a mercury-in-glass thermometer or the pyrometric sensor in an infrared thermometer) in which some change occurs with a change in temperature; and (2) some means of converting this change into a numerical value (e.g. the visible scale that is marked on a mercury-in-glass thermometer or the digital readout on an infrared model). Thermometers are widely used in technology and industry to monitor processes, in meteorology, in medicine, and in scientific research. History While an individual thermometer is able to measure degrees of hotness, the readings on two thermometers cannot be compared unless they conform to an agreed scale. Today there is an absolute thermodynamic temperature scale. Internationally agreed temperature scales are designed to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Latent Heat
Latent heat (also known as latent energy or heat of transformation) is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process — usually a first-order phase transition. Latent heat can be understood as energy in hidden form which is supplied or extracted to change the state of a substance without changing its temperature. Examples are latent heat of fusion and latent heat of vaporization involved in phase changes, i.e. a substance condensing or vaporizing at a specified temperature and pressure. The term was introduced around 1762 by Scottish chemist Joseph Black. It is derived from the Latin ''latere'' (''to lie hidden''). Black used the term in the context of calorimetry where a heat transfer caused a volume change in a body while its temperature was constant. In contrast to latent heat, sensible heat is energy transferred as heat, with a resultant temperature change in a body. Usage The terms ″sensible heat″ and ″laten ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Heat
In thermodynamics, the specific heat capacity (symbol ) of a substance is the heat capacity of a sample of the substance divided by the mass of the sample, also sometimes referred to as massic heat capacity. Informally, it is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. The SI unit of specific heat capacity is joule per kelvin per kilogram, J⋅kg−1⋅K−1. For example, the heat required to raise the temperature of of water by is , so the specific heat capacity of water is . Specific heat capacity often varies with temperature, and is different for each state of matter. Liquid water has one of the highest specific heat capacities among common substances, about at 20 °C; but that of ice, just below 0 °C, is only . The specific heat capacities of iron, granite, and hydrogen gas are about 449 J⋅kg−1⋅K−1, 790 J⋅kg−1⋅K−1, and 14300 J⋅kg−1⋅K−1 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not consi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atmospheric Pressure
Atmospheric pressure, also known as barometric pressure (after the barometer), is the pressure within the atmosphere of Earth. The standard atmosphere (symbol: atm) is a unit of pressure defined as , which is equivalent to 1013.25 millibars, 760mm Hg, 29.9212 inchesHg, or 14.696psi.International Civil Aviation Organization. ''Manual of the ICAO Standard Atmosphere'', Doc 7488-CD, Third Edition, 1993. . The atm unit is roughly equivalent to the mean sea-level atmospheric pressure on Earth; that is, the Earth's atmospheric pressure at sea level is approximately 1 atm. In most circumstances, atmospheric pressure is closely approximated by the hydrostatic pressure caused by the weight of air above the measurement point. As elevation increases, there is less overlying atmospheric mass, so atmospheric pressure decreases with increasing elevation. Because the atmosphere is thin relative to the Earth's radius—especially the dense atmospheric layer at low altitudes—the Earth's gravi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Altitude
Altitude or height (also sometimes known as depth) is a distance measurement, usually in the vertical or "up" direction, between a reference datum and a point or object. The exact definition and reference datum varies according to the context (e.g., aviation, geometry, geographical survey, sport, or atmospheric pressure). Although the term ''altitude'' is commonly used to mean the height above sea level of a location, in geography the term elevation is often preferred for this usage. Vertical distance measurements in the "down" direction are commonly referred to as depth. In aviation In aviation, the term altitude can have several meanings, and is always qualified by explicitly adding a modifier (e.g. "true altitude"), or implicitly through the context of the communication. Parties exchanging altitude information must be clear which definition is being used. Aviation altitude is measured using either mean sea level (MSL) or local ground level (above ground level, or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dewpoint
The dew point is the temperature to which air must be cooled to become saturated with water vapor, assuming constant air pressure and water content. When cooled below the dew point, moisture capacity is reduced and airborne water vapor will condense to form liquid water known as dew. When this occurs via contact with a colder surface, dew will form on that surface. The dew point is affected by humidity. When there is more moisture in the air, the dew point is higher. When the temperature is below the freezing point of water, the dew point is called the frost point, as frost is formed via deposition rather than condensation. In liquids, the analog to the dew point is the cloud point. Humidity If all the other factors influencing humidity remain constant, at ground level the relative humidity rises as the temperature falls; this is because less vapor is needed to saturate the air. In normal conditions, the dew point temperature will not be greater than the air temperature, sinc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Properties
A chemical property is any of a material's properties that becomes evident during, or after, a chemical reaction; that is, any quality that can be established only by changing a substance's chemical identity.William L. Masterton, Cecile N. Hurley, "Chemistry: Principles and Reactions", 6th edition. Brooks/Cole Cengage Learning, 2009, p.1(Google books)/ref> Simply speaking, chemical properties cannot be determined just by viewing or touching the substance; the substance's internal structure must be affected greatly for its chemical properties to be investigated. When a substance goes under a chemical reaction, the properties will change drastically, resulting in chemical change. However, a catalytic property would also be a chemical property. Chemical properties can be contrasted with physical properties, which can be discerned without changing the substance's structure. However, for many properties within the scope of physical chemistry, and other disciplines at the boundary betw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Laws
The gas laws were developed at the end of the 18th century, when scientists began to realize that relationships between pressure, volume and temperature of a sample of gas could be obtained which would hold to approximation for all gases. Boyle's law In 1662 Robert Boyle studied the relationship between volume and pressure of a gas of fixed amount at constant temperature. He observed that volume of a given mass of a gas is inversely proportional to its pressure at a constant temperature. Boyle's law, published in 1662, states that, at constant temperature, the product of the pressure and volume of a given mass of an ideal gas in a closed system is always constant. It can be verified experimentally using a pressure gauge and a variable volume container. It can also be derived from the kinetic theory of gases: if a container, with a fixed number of molecules inside, is reduced in volume, more molecules will strike a given area of the sides of the container per unit time, causing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Engineering Thermodynamics
A chemical substance is a form of matter having constant chemical composition and characteristic properties. Some references add that chemical substance cannot be separated into its constituent elements by physical separation methods, i.e., without breaking chemical bonds. Chemical substances can be simple substances (substances consisting of a single chemical element), chemical compounds, or alloys. Chemical substances are often called 'pure' to set them apart from mixtures. A common example of a chemical substance is pure water; it has the same properties and the same ratio of hydrogen to oxygen whether it is isolated from a river or made in a laboratory. Other chemical substances commonly encountered in pure form are diamond (carbon), gold, table salt ( sodium chloride) and refined sugar ( sucrose). However, in practice, no substance is entirely pure, and chemical purity is specified according to the intended use of the chemical. Chemical substances exist as solids, liqu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |