|
Plumbylene Structure
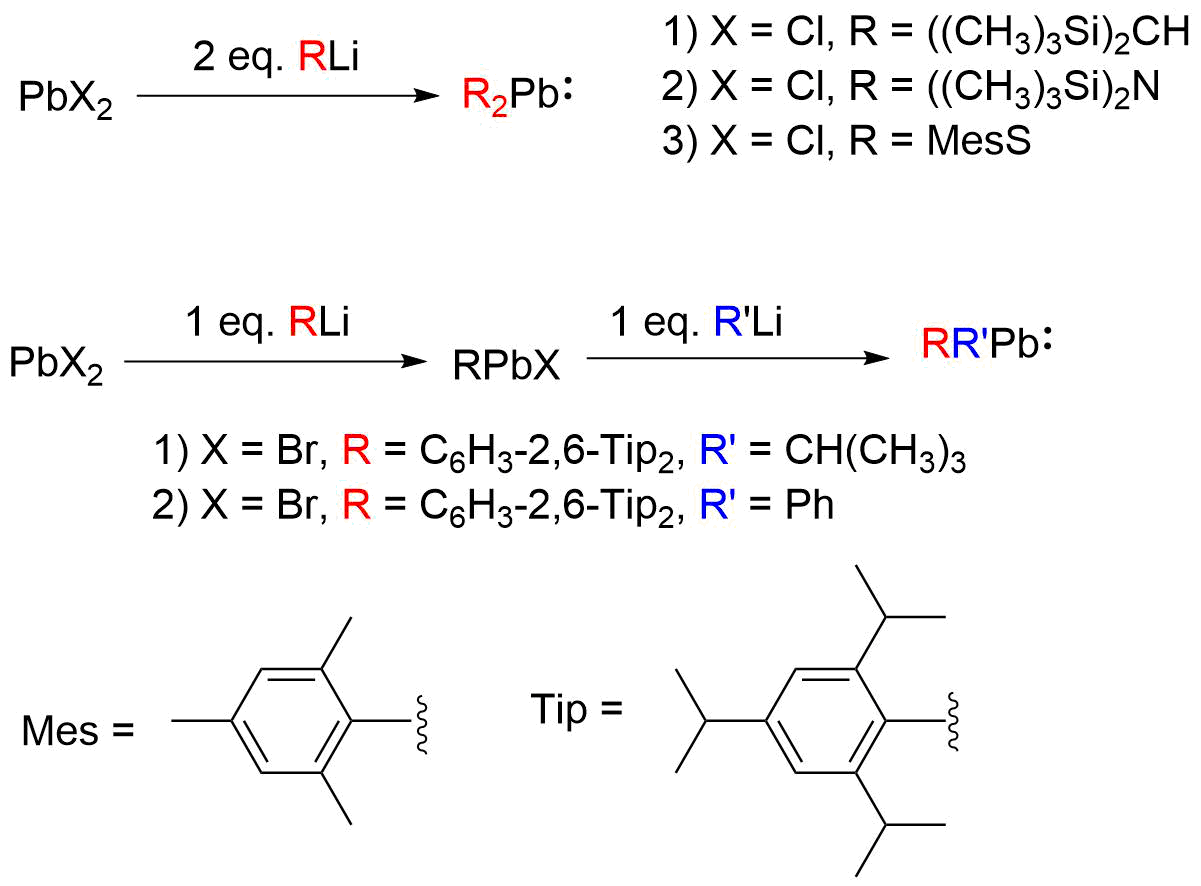
Plumbylenes (or plumbylidenes) are divalent organolead(II) analogues of carbenes, with the general chemical formula, R2Pb, where R denotes a substituent. Plumbylenes possess 6 electrons in their valence shell, and are considered open shell species. The first plumbylene reported was the dialkylplumbylene, Me3Si)2CHsub>2Pb, which was synthesized by Michael F. Lappert ''et al'' in 1973. Plumbylenes may be further classified into carbon-substituted plumbylenes, plumbylenes stabilized by a group 15 or 16 element, and monohalogenated plumbylenes (RPbX). Synthesis Plumbylenes can generally be synthesized via the transmetallation of PbX2 (where X denotes halogen) with an organolithium (RLi) or Grignard reagent (RMgX). The first reported plumbylene, (CH3)3Si)2CHsub>2Pb, was synthesized by Michael F. Lappert ''et al'' by transmetallation of PbCl2 with (CH3)3Si)2CHi. The addition of equimolar RLi to PbX2 produces the monohalogenated plumbylene (RPbX); addition of 2 equivalents leads ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plumbylene Structure
Plumbylenes (or plumbylidenes) are divalent organolead(II) analogues of carbenes, with the general chemical formula, R2Pb, where R denotes a substituent. Plumbylenes possess 6 electrons in their valence shell, and are considered open shell species. The first plumbylene reported was the dialkylplumbylene, Me3Si)2CHsub>2Pb, which was synthesized by Michael F. Lappert ''et al'' in 1973. Plumbylenes may be further classified into carbon-substituted plumbylenes, plumbylenes stabilized by a group 15 or 16 element, and monohalogenated plumbylenes (RPbX). Synthesis Plumbylenes can generally be synthesized via the transmetallation of PbX2 (where X denotes halogen) with an organolithium (RLi) or Grignard reagent (RMgX). The first reported plumbylene, (CH3)3Si)2CHsub>2Pb, was synthesized by Michael F. Lappert ''et al'' by transmetallation of PbCl2 with (CH3)3Si)2CHi. The addition of equimolar RLi to PbX2 produces the monohalogenated plumbylene (RPbX); addition of 2 equivalents leads ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plumbylene Synthesis Transmetallation
Plumbylenes (or plumbylidenes) are divalent organolead(II) analogues of carbenes, with the general chemical formula, R2Pb, where R denotes a substituent. Plumbylenes possess 6 electrons in their valence shell, and are considered open shell species. The first plumbylene reported was the dialkylplumbylene, Me3Si)2CHsub>2Pb, which was synthesized by Michael F. Lappert ''et al'' in 1973. Plumbylenes may be further classified into carbon-substituted plumbylenes, plumbylenes stabilized by a group 15 or 16 element, and monohalogenated plumbylenes (RPbX). Synthesis Plumbylenes can generally be synthesized via the transmetallation of PbX2 (where X denotes halogen) with an organolithium (RLi) or Grignard reagent (RMgX). The first reported plumbylene, (CH3)3Si)2CHsub>2Pb, was synthesized by Michael F. Lappert ''et al'' by transmetallation of PbCl2 with (CH3)3Si)2CHi. The addition of equimolar RLi to PbX2 produces the monohalogenated plumbylene (RPbX); addition of 2 equivalents leads ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
A European Journal
A, or a, is the first letter and the first vowel of the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''a'' (pronounced ), plural ''aes''. It is similar in shape to the Ancient Greek letter alpha, from which it derives. The uppercase version consists of the two slanting sides of a triangle, crossed in the middle by a horizontal bar. The lowercase version can be written in two forms: the double-storey a and single-storey ɑ. The latter is commonly used in handwriting and fonts based on it, especially fonts intended to be read by children, and is also found in italic type. In English grammar, " a", and its variant " an", are indefinite articles. History The earliest certain ancestor of "A" is aleph (also written 'aleph), the first letter of the Phoenician alphabet, which consisted entirely of consonants (for that reason, it is also called an abjad to distinguish ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Angle
Bond or bonds may refer to: Common meanings * Bond (finance), a type of debt security * Bail bond, a commercial third-party guarantor of surety bonds in the United States * Chemical bond, the attraction of atoms, ions or molecules to form chemical compounds People * Bond (surname) * Bonds (surname) * Mr. Bond (musician), Austrian rapper Arts and entertainment * James Bond, a series of works about the eponymous fictional character * James Bond (literary character), a British secret agent in a series of novels and films * Bond (band), an Australian/British string quartet ** '' Bond: Video Clip Collection'', a video collection from the band * Bond (Canadian band), a Canadian rock band in the 1970s * ''The Bond'' (2007 book), an American autobiography written by The Three Doctors * ''The Bond'', a 1918 film by Charlie Chaplin supporting Liberty bonds * Bond International Casino, a former music venue in New York City Places Antarctica * Bond Glacier, at the head of Vincennes B ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule. Explanation Bond length is related to bond order: when more electrons participate in bond formation the bond is shorter. Bond length is also inversely related to bond strength and the bond dissociation energy: all other factors being equal, a stronger bond will be shorter. In a bond between two identical atoms, half the bond distance is equal to the covalent radius. Bond lengths are measured in the solid phase by means of X-ray diffraction, or approximated in the gas phase by microwave spectroscopy. A bond between a given pair of atoms may vary between different molecules. For example, the carbon to hydrogen bonds in methane are different from those in methyl chloride. It is however possible to make generalizations when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ground State
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. In quantum field theory, the ground state is usually called the vacuum state or the vacuum. If more than one ground state exists, they are said to be degenerate. Many systems have degenerate ground states. Degeneracy occurs whenever there exists a unitary operator that acts non-trivially on a ground state and commutes with the Hamiltonian of the system. According to the third law of thermodynamics, a system at absolute zero temperature exists in its ground state; thus, its entropy is determined by the degeneracy of the ground state. Many systems, such as a perfect crystal lattice, have a unique ground state and therefore have zero entropy at absolute zero. It is also possible for the highest excited state to have absolute zero temper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spin State
Spin or spinning most often refers to: * Spinning (textiles), the creation of yarn or thread by twisting fibers together, traditionally by hand spinning * Spin, the rotation of an object around a central axis * Spin (propaganda), an intentionally biased portrayal of something Spin, spinning or spinnin may also refer to: Physics and mathematics * Spin, the rotation of an object around a central axis * Spin (physics) or particle spin, a fundamental property of elementary particles * Spin group, a particular double cover of the special orthogonal group SO(''n'') * Spin tensor, a tensor quantity for describing spinning motion in special relativity and general relativity * Spin (aerodynamics), autorotation of an aerodynamically stalled aeroplane * SPIN bibliographic database, an indexing and abstracting service focusing on physics research Textile arts * Spinning (polymers), a process for creating polymer fibres * Spinning (textiles), the creation of yarn or thread by twisting fibe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sp Hybridization
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed out that a carbon atom forms four b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Relativistic Effect
Relativistic quantum chemistry combines relativistic mechanics with quantum chemistry to calculate elemental properties and structure, especially for the heavier elements of the periodic table. A prominent example is an explanation for the color of gold: due to relativistic effects, it is not silvery like most other metals. The term ''relativistic effects'' were developed in light of the history of quantum mechanics. Initially, quantum mechanics was developed without considering the theory of relativity. Relativistic effects are those discrepancies between values calculated by models that consider relativity and those that do not. Relativistic effects are important for heavier elements with high atomic numbers, such as lanthanides and actinides. Relativistic effects in chemistry can be considered to be perturbations, or small corrections, to the non-relativistic theory of chemistry, which is developed from the solutions of the Schrödinger equation. These corrections affect the el ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Group 14
The carbon group is a group (periodic table), periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block. In modern International Union of Pure and Applied Chemistry, IUPAC notation, it is called group 14. In the field of Semiconductor#Physics of semiconductors, semiconductor physics, it is still universally called group IV. The group was once also known as the tetrels (from the Greek word ''tetra'', which means four), stemming from the Roman numeral IV in the group names, or (not coincidentally) from the fact that these elements have four valence electrons (see below). They are also known as the crystallogens or adamantogens. Characteristics Chemical Like other groups, the members of this family show patterns in electron configuration, especially in the outermost shells, resulting in trends in chemical behavior: Each of the chemical element, elements in this group has 4 electrons in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Gap
In solid-state physics, an energy gap is an energy range in a solid where no electron states exist, i.e. an energy range where the density of states vanishes. Especially in condensed-matter physics, an energy gap is often known more abstractly as a spectral gap, a term which need not be specific to electrons or solids. Band gap If an energy gap exists in the band structure of a material, it is called band gap. The physical properties of semiconductors are to a large extent determined by their band gaps, but also for insulators and metals the band structure—and thus any possible band gaps—govern their electronic properties. Superconductors For superconductors the energy gap is a region of suppressed density of states around the Fermi energy, with the size of the energy gap much smaller than the energy scale of the band structure. The superconducting energy gap is a key aspect in the theoretical description of superconductivity and thus features prominently in BCS theory. Her ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |