|
Group 14
The carbon group is a periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block. In modern IUPAC notation, it is called group 14. In the field of semiconductor physics, it is still universally called group IV. The group was once also known as the tetrels (from the Greek word ''tetra'', which means four), stemming from the Roman numeral IV in the group names, or (not coincidentally) from the fact that these elements have four valence electrons (see below). They are also known as the crystallogens or adamantogens. Characteristics Chemical Like other groups, the members of this family show patterns in electron configuration, especially in the outermost shells, resulting in trends in chemical behavior: Each of the elements in this group has 4 electrons in its outer shell. An isolated, neutral group 14 atom has the s2 p2 configuration in the ground state. These elements, especial ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Group (periodic Table)
In chemistry, a group (also known as a family) is a column of elements in the periodic table of the chemical elements. There are 18 numbered groups in the periodic table; the f-block columns (between groups 2 and 3) are not numbered. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms (i.e., the same core charge), because most chemical properties are dominated by the orbital location of the outermost electron. There are three systems of group numbering for the groups; the same number may be assigned to different groups depending on the system being used. The modern numbering system of "group 1" to "group 18" has been recommended by the International Union of Pure and Applied Chemistry (IUPAC) since about 1990. It replaces two older incompatible naming schemes, used by the Chemical Abstract Service (CAS, more popular in the US), and by IUPAC before 1990 (more popular in Europe). The system of eighteen group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lead
Lead is a chemical element with the Symbol (chemistry), symbol Pb (from the Latin ) and atomic number 82. It is a heavy metals, heavy metal that is density, denser than most common materials. Lead is Mohs scale of mineral hardness#Intermediate hardness, soft and malleable, and also has a relatively low melting point. When freshly cut, lead is a shiny gray with a hint of blue. It tarnishes to a dull gray color when exposed to air. Lead has the highest atomic number of any stable nuclide, stable element and three of its isotopes are endpoints of major nuclear decay chains of heavier elements. Lead is toxic, even in small amounts, especially to children. Lead is a relatively unreactive post-transition metal. Its weak metallic character is illustrated by its amphoteric nature; lead and lead oxides react with acids and base (chemistry), bases, and it tends to form covalent bonds. Compounds of lead are usually found in the +2 oxidation state rather than the +4 state common with lighte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pi Bond
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals has an electron density of zero at a shared nodal plane that passes through the two bonded nuclei. This plane also is a nodal plane for the molecular orbital of the pi bond. Pi bonds can form in double and triple bonds but do not form in single bonds in most cases. The Greek letter π in their name refers to p orbitals, since the orbital symmetry of the pi bond is the same as that of the p orbital when seen down the bond axis. One common form of this sort of bonding involves p orbitals themselves, though d orbitals also engage in pi bonding. This latter mode forms part of the basis for metal-metal multiple bonding. Pi bonds are usually weaker than sigma bonds. The C-C double bond, composed of one sigma and one pi bond, ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
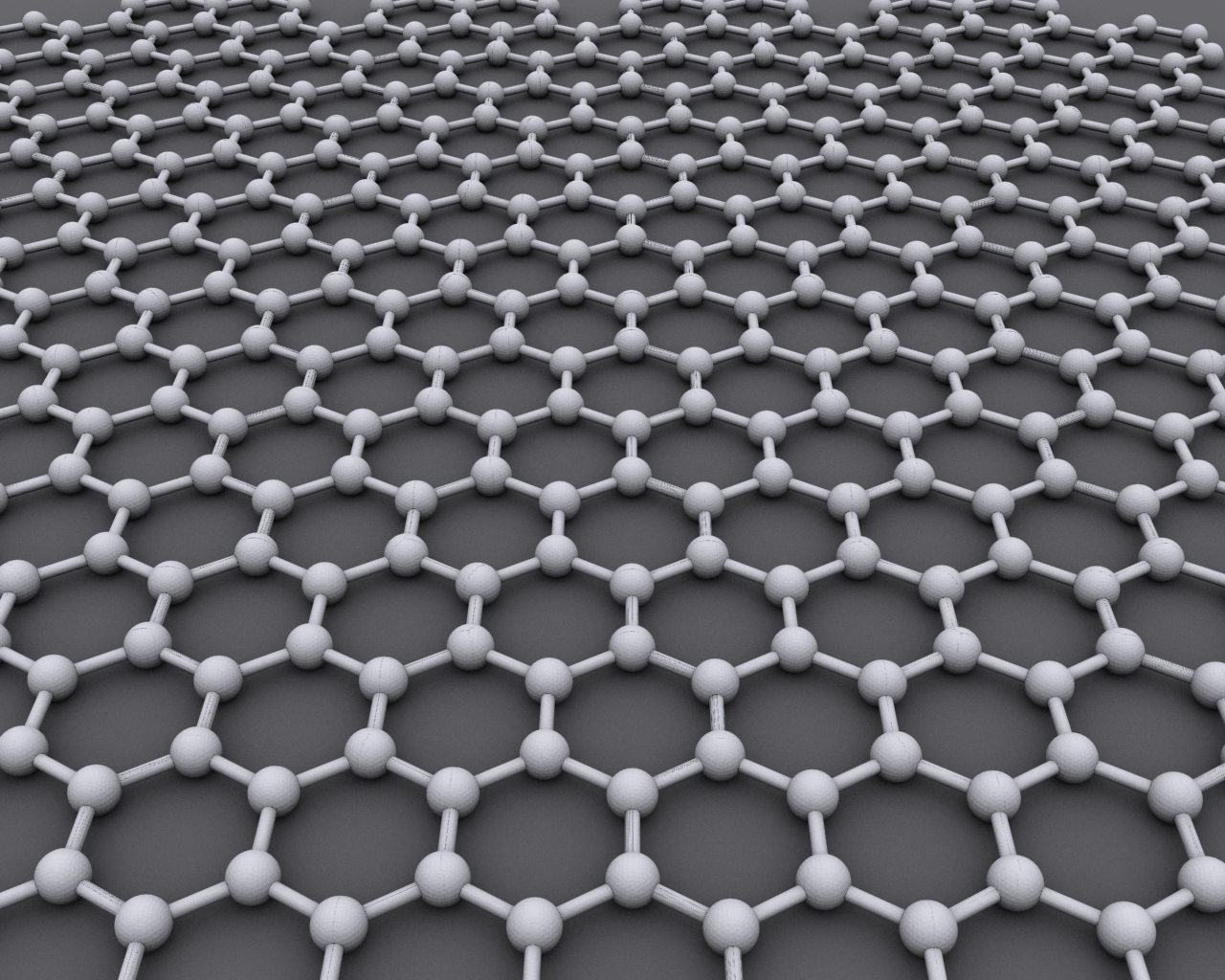
Graphene
Graphene () is an allotrope of carbon consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice nanostructure. "Carbon nanostructures for electromagnetic shielding applications", Mohammed Arif Poothanari, Sabu Thomas, et al., ''Industrial Applications of Nanomaterials'', 2019. "Carbon nanostructures include various low-dimensional allotropes of carbon including carbon black (CB), carbon fiber, carbon nanotubes (CNTs), fullerene, and graphene." The name is derived from "graphite" and the suffix -ene, reflecting the fact that the graphite allotrope of carbon contains numerous double bonds. Each atom in a graphene sheet is connected to its three nearest neighbors by a strong σ-bond, and contributes to a valence band one electron that extends over the whole sheet. This is the same type of b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahedral Molecular Geometry
In a tetrahedral molecular geometry, a central atom is located at the center with four substituents that are located at the corners of a tetrahedron. The bond angles are cos−1(−) = 109.4712206...° ≈ 109.5° when all four substituents are the same, as in methane () as well as its heavier analogues. Methane and other perfectly symmetrical tetrahedral molecules belong to point group Td, but most tetrahedral molecules have lower symmetry. Tetrahedral molecules can be chiral. Tetrahedral bond angle The bond angle for a symmetric tetrahedral molecule such as CH4 may be calculated using the dot product of two vectors. As shown in the diagram, the molecule can be inscribed in a cube with the tetravalent atom (e.g. carbon) at the cube centre which is the origin of coordinates, O. The four monovalent atoms (e.g. hydrogens) are at four corners of the cube (A, B, C, D) chosen so that no two atoms are at adjacent corners linked by only one cube edge. If the edge length of the cube ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Single Bond
In chemistry, a single bond is a chemical bond between two atoms involving two valence electrons. That is, the atoms share one pair of electrons where the bond forms. Therefore, a single bond is a type of covalent bond. When shared, each of the two electrons involved is no longer in the sole possession of the orbital in which it originated. Rather, both of the two electrons spend time in either of the orbitals which overlap in the bonding process. As a Lewis structure, a single bond is denoted as AːA or A-A, for which A represents an element. In the first rendition, each dot represents a shared electron, and in the second rendition, the bar represents both of the electrons shared in the single bond. A covalent bond can also be a double bond or a triple bond. A single bond is weaker than either a double bond or a triple bond. This difference in strength can be explained by examining the component bonds of which each of these types of covalent bonds consists (Moore, Stanitski, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
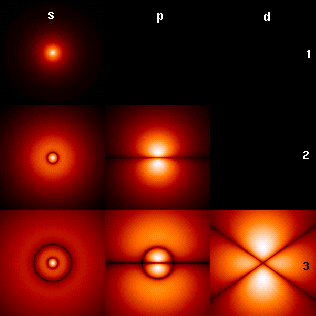
Azimuthal Quantum Number
The azimuthal quantum number is a quantum number for an atomic orbital that determines its angular momentum operator, orbital angular momentum and describes the shape of the orbital. The wikt:azimuthal, azimuthal quantum number is the second of a set of quantum numbers that describe the unique quantum state of an electron (the others being the principal quantum number, the magnetic quantum number, and the spin quantum number). It is also known as the orbital angular momentum quantum number, orbital quantum number or second quantum number, and is symbolized as ℓ (pronounced ''ell''). Derivation Connected with the energy states of the atom's electrons are four quantum numbers: ''n'', ''ℓ'', ''m''''ℓ'', and ''m''''s''. These specify the complete, unique quantum state of a single electron in an atom, and make up its wavefunction or ''orbital''. When solving to obtain the wave function, the Schrödinger equation reduces to three equations that lead to the first three quantum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orbital Hybridisation
In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new ''hybrid orbitals'' (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines with three valence-shell p orbitals to form four equivalent sp3 mixtures in a tetrahedral arrangement around the carbon to bond to four different atoms. Hybrid orbitals are useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space. Usually hybrid orbitals are formed by mixing atomic orbitals of comparable energies. History and uses Chemist Linus Pauling first developed the hybridisation theory in 1931 to explain the structure of simple molecules such as methane (CH4) using atomic orbitals. Pauling pointed out that a carbon atom forms fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octet Rule
The octet rule is a chemical rule of thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rule is especially applicable to carbon, nitrogen, oxygen, and the halogens; although more generally the rule is applicable for the s-block and p-block of the periodic table. Other rules exist for other elements, such as the duplet rule for hydrogen and helium, or the 18-electron rule for transition metals. The valence electrons can be counted using a Lewis structure, Lewis electron dot diagram as shown at the right for carbon dioxide. The electrons shared by the two atoms in a covalent bond are counted twice, once for each atom. In carbon dioxide each oxygen shares four electrons with the central carbon, two (shown in red) from the oxygen itself and two (shown in black) from the carbon. All four of these electrons are counted in bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Covalent Bond
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence shell, corresponding to a stable electronic configuration. In organic chemistry, covalent bonding is much more common than ionic bonding. Covalent bonding also includes many kinds of interactions, including σ-bonding, π-bonding, metal-to-metal bonding, agostic interactions, bent bonds, three-center two-electron bonds and three-center four-electron bonds. The term ''covalent bond'' dates from 1939. The prefix ''co-'' means ''jointly, associated in action, partnered to a lesser degree, '' etc.; thus a "co-valent bond", in essence, means that the atoms share "valence" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Shell
In chemistry and atomic physics, an electron shell may be thought of as an orbit followed by electrons around an atom's Atomic nucleus, nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (or "L shell"), then the "3 shell" (or "M shell"), and so on farther and farther from the nucleus. The shells correspond to the principal quantum numbers (''n'' = 1, 2, 3, 4 ...) or are labeled alphabetically with the letters used in X-ray notation (K, L, M, ...). A useful guide when understanding electron shells in atoms is to note that each row on the conventional periodic table of elements represents an electron shell. Each shell can contain only a fixed number of electrons: the first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on. The general formula is that the ''n''th shell can in principle hold up to 2(square ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |