Plumbylene Structure on:
[Wikipedia]
[Google]
[Amazon]
 Plumbylenes (or plumbylidenes) are divalent organolead(II) analogues of
Plumbylenes (or plumbylidenes) are divalent organolead(II) analogues of
 Transmetallation with (CH3)3Si)2Nsub>2Pb as the Pb(II) precursor has also been used to synthesize diarylplumbylenes, disilylplumbylenes, and saturated ''N''-heterocyclic plumbylenes.
Transmetallation with (CH3)3Si)2Nsub>2Pb as the Pb(II) precursor has also been used to synthesize diarylplumbylenes, disilylplumbylenes, and saturated ''N''-heterocyclic plumbylenes.
 Alternatively, plumbylenes may be synthesized from the reductive dehalogenation of tetravalent organolead compounds (R2PbX2).
Alternatively, plumbylenes may be synthesized from the reductive dehalogenation of tetravalent organolead compounds (R2PbX2).

 The key aspects of bonding and reactivity in plumbylenes are dictated by the
The key aspects of bonding and reactivity in plumbylenes are dictated by the  Diphenyllead, (C6H5)2Pb was computed with GAMESS at the B3PW91 level of theory using the basis sets 6-311+G(2df,p) for C and H and def2-svp for Pb with the ECP60MDF pseudopotential, in an adapted procedure (which uses the cc-pVTZ basis set for Pb instead). The molecular orbitals (MOs) (visualized using Chimera) and natural bond orbitals (NBOs) (visualized using multiwfn) generated are produced below, and qualitatively identical to the literature. As expected, the HOMO is 6s-dominated, and the LUMO is 6p-dominated. The NBOs are of the 6s lone pair and vacant 6p orbital respectively.
Diphenyllead, (C6H5)2Pb was computed with GAMESS at the B3PW91 level of theory using the basis sets 6-311+G(2df,p) for C and H and def2-svp for Pb with the ECP60MDF pseudopotential, in an adapted procedure (which uses the cc-pVTZ basis set for Pb instead). The molecular orbitals (MOs) (visualized using Chimera) and natural bond orbitals (NBOs) (visualized using multiwfn) generated are produced below, and qualitatively identical to the literature. As expected, the HOMO is 6s-dominated, and the LUMO is 6p-dominated. The NBOs are of the 6s lone pair and vacant 6p orbital respectively.
The Pb–C bond distance was found to be 2.303 Å and the C–Pb–C angle 105.7°. Notwithstanding the different levels of theory, the larger bond angle for (C6H5)2Pb compared to can be rationalized by the greater repulsion between the sterically bulkier phenyl groups relative to methyl groups. Atoms in molecules (AIM) topology analysis revealed critical points in (C6H5)2Pb, and is consistent with the literature. Plumbylenes occur as reactive intermediates in the formation of tetravalent
Plumbylenes occur as reactive intermediates in the formation of tetravalent
 Plumbylenes are able to undergo dimerization in two ways: either through the formation of a Pb=Pb double bond to form a formal diplumbene, or through bridging halide interactions. Unhalogenated plumbylenes tend to exist in an equilibrium between the monomeric and dimeric form in solution, and, due to the low dimerization energy, as either monomers or dimers in the solid state, depending on the steric bulk of substituents. However, increasing the steric bulk of lead-bound substituents can prevent the close association of plumbylene molecules and allow the plumbylene to exist exclusively as monomers in solution or even in the solid state.
The driving force for dimerization in general arises from the Lewis amphoteric nature of plumbylenes, which possess a
Plumbylenes are able to undergo dimerization in two ways: either through the formation of a Pb=Pb double bond to form a formal diplumbene, or through bridging halide interactions. Unhalogenated plumbylenes tend to exist in an equilibrium between the monomeric and dimeric form in solution, and, due to the low dimerization energy, as either monomers or dimers in the solid state, depending on the steric bulk of substituents. However, increasing the steric bulk of lead-bound substituents can prevent the close association of plumbylene molecules and allow the plumbylene to exist exclusively as monomers in solution or even in the solid state.
The driving force for dimerization in general arises from the Lewis amphoteric nature of plumbylenes, which possess a  These diplumbenes possess a ''trans''-bent structure similar to that in lighter, non-carbon congeners (
These diplumbenes possess a ''trans''-bent structure similar to that in lighter, non-carbon congeners (
 In a recent study, an ''N''-heterocyclic plumbylene was shown to undergo dimerization leading to C–H activation, existing in solution in an equilibrium between the monomer and a dimer resulting from cleavage of an aryl C–H bond and formation of Pb–C and N–H bonds. DFT studies proposed that the reaction occurred via electrophilic substitution at the arene of one plumbylene by the lead atom of another, and involves concerted Pb–C and N–H bond formation instead of insertion of Pb into the C–H bond.
In a recent study, an ''N''-heterocyclic plumbylene was shown to undergo dimerization leading to C–H activation, existing in solution in an equilibrium between the monomer and a dimer resulting from cleavage of an aryl C–H bond and formation of Pb–C and N–H bonds. DFT studies proposed that the reaction occurred via electrophilic substitution at the arene of one plumbylene by the lead atom of another, and involves concerted Pb–C and N–H bond formation instead of insertion of Pb into the C–H bond.

 Insertions into lead-substituent bonds can also occur.27 In the examples below, insertion is accompanied by intramolecular rearrangement to place more electron-donating heteroatoms next to the electron-deficient lead.27
Insertions into lead-substituent bonds can also occur.27 In the examples below, insertion is accompanied by intramolecular rearrangement to place more electron-donating heteroatoms next to the electron-deficient lead.27

 Plumbylenes can be used as concurrent σ-donor-σ-acceptor
Plumbylenes can be used as concurrent σ-donor-σ-acceptor
carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
s, with the general chemical formula, R2Pb, where R denotes a substituent. Plumbylenes possess 6 electrons in their valence shell
In chemistry and physics, a valence electron is an electron in the outer shell associated with an atom, and that can participate in the formation of a chemical bond if the outer shell is not closed. In a single covalent bond, a shared pair forms ...
, and are considered open shell
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom ...
species.
The first plumbylene reported was the dialkylplumbylene, Me3Si)2CHsub>2Pb, which was synthesized by Michael F. Lappert
Michael Franz Lappert (31 December 1928 – 28 March 2014) was a Czech-born British inorganic chemist. Mainly located at the University of Sussex, he was recognized for contributions to organometallic complexes.
Early life and education
Lapp ...
''et al'' in 1973.
Plumbylenes may be further classified into carbon-substituted plumbylenes, plumbylenes stabilized by a group 15
A pnictogen ( or ; from grc, πνῑ́γω "to choke" and -gen, "generator") is any of the chemical elements in group 15 of the periodic table. Group 15 is also known as the nitrogen group or nitrogen family. Group 15 consists of the el ...
or 16 element, and monohalogenated plumbylenes (RPbX).
Synthesis
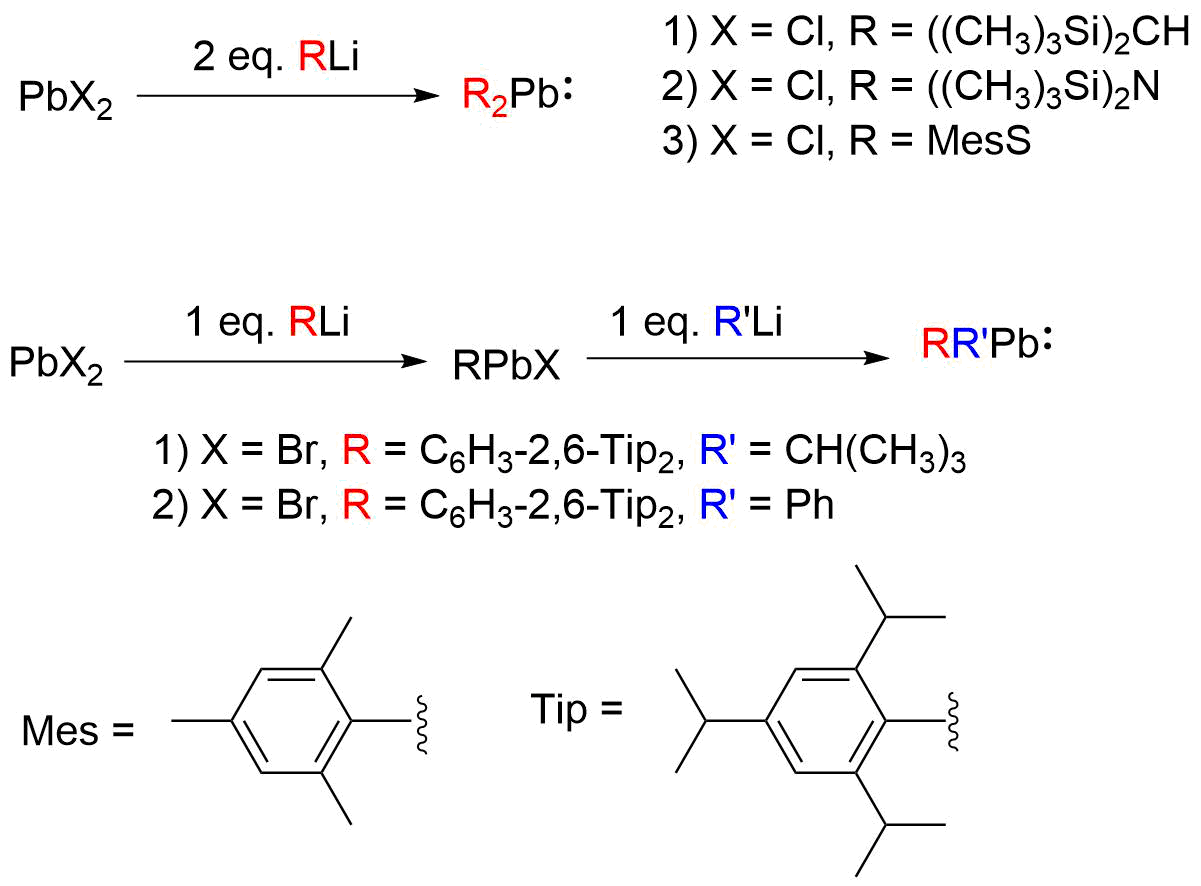
Plumbylenes can generally be synthesized via thetransmetallation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
of PbX2 (where X denotes halogen) with an organolithium (RLi) or Grignard reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide ...
(RMgX). The first reported plumbylene, (CH3)3Si)2CHsub>2Pb, was synthesized by Michael F. Lappert
Michael Franz Lappert (31 December 1928 – 28 March 2014) was a Czech-born British inorganic chemist. Mainly located at the University of Sussex, he was recognized for contributions to organometallic complexes.
Early life and education
Lapp ...
''et al'' by transmetallation of PbCl2 with (CH3)3Si)2CHi. The addition of equimolar RLi to PbX2 produces the monohalogenated plumbylene (RPbX); addition of 2 equivalents leads to disubstituted plumbylene (R2Pb). Adding an organolithium or Grignard reagent with a different organic substituent (i.e. R’Li/R’MgX) from RPbX leads to the synthesis of heteroleptic plumbylenes (RR’Pb). Dialkyl-, diaryl-, diamido-, dithioplumbylenes, and monohalogenated plumbyelenes have been successfully synthesized this way. Transmetallation with (CH3)3Si)2Nsub>2Pb as the Pb(II) precursor has also been used to synthesize diarylplumbylenes, disilylplumbylenes, and saturated ''N''-heterocyclic plumbylenes.
Transmetallation with (CH3)3Si)2Nsub>2Pb as the Pb(II) precursor has also been used to synthesize diarylplumbylenes, disilylplumbylenes, and saturated ''N''-heterocyclic plumbylenes.
 Alternatively, plumbylenes may be synthesized from the reductive dehalogenation of tetravalent organolead compounds (R2PbX2).
Alternatively, plumbylenes may be synthesized from the reductive dehalogenation of tetravalent organolead compounds (R2PbX2).

Structure and bonding
inert pair effect The inert-pair effect is the tendency of the two electrons in the outermost atomic ''s''-orbital to remain unshared in compounds of post-transition metals. The term ''inert-pair effect'' is often used in relation to the increasing stability of oxi ...
, whereby the combination of a widening s–p orbital energy gap
In solid-state physics, an energy gap is an energy range in a solid where no electron states exist, i.e. an energy range where the density of states vanishes.
Especially in condensed-matter physics, an energy gap is often known more abstractly as ...
as a trend down the group 14
The carbon group is a group (periodic table), periodic table group consisting of carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). It lies within the p-block.
In modern International Union of Pure and Applied Chem ...
elements and a strong relativistic contraction of the 6s orbital lead to a limited degree of sp hybridization and the 6s orbital being deep in energy and inert. Consequently, plumbylenes exclusively have a singlet spin state due to the large singlet–triplet energy gap, and tend to exist in an equilibrium between monomeric and dimeric forms in solution. This is in contrast to carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" ma ...
s, which often have a triplet ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state. ...
and readily dimerize to form alkenes.
In dimethyllead, (CH3)2Pb, the Pb–C bond length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of ...
is 2.267 Å and the C–Pb–C bond angle
Bond or bonds may refer to:
Common meanings
* Bond (finance), a type of debt security
* Bail bond, a commercial third-party guarantor of surety bonds in the United States
* Chemical bond, the attraction of atoms, ions or molecules to form chemical ...
is 93.02°; the singlet–triplet gap is 36.99 kcal mol−1.
 Diphenyllead, (C6H5)2Pb was computed with GAMESS at the B3PW91 level of theory using the basis sets 6-311+G(2df,p) for C and H and def2-svp for Pb with the ECP60MDF pseudopotential, in an adapted procedure (which uses the cc-pVTZ basis set for Pb instead). The molecular orbitals (MOs) (visualized using Chimera) and natural bond orbitals (NBOs) (visualized using multiwfn) generated are produced below, and qualitatively identical to the literature. As expected, the HOMO is 6s-dominated, and the LUMO is 6p-dominated. The NBOs are of the 6s lone pair and vacant 6p orbital respectively.
Diphenyllead, (C6H5)2Pb was computed with GAMESS at the B3PW91 level of theory using the basis sets 6-311+G(2df,p) for C and H and def2-svp for Pb with the ECP60MDF pseudopotential, in an adapted procedure (which uses the cc-pVTZ basis set for Pb instead). The molecular orbitals (MOs) (visualized using Chimera) and natural bond orbitals (NBOs) (visualized using multiwfn) generated are produced below, and qualitatively identical to the literature. As expected, the HOMO is 6s-dominated, and the LUMO is 6p-dominated. The NBOs are of the 6s lone pair and vacant 6p orbital respectively.The Pb–C bond distance was found to be 2.303 Å and the C–Pb–C angle 105.7°. Notwithstanding the different levels of theory, the larger bond angle for (C6H5)2Pb compared to can be rationalized by the greater repulsion between the sterically bulkier phenyl groups relative to methyl groups. Atoms in molecules (AIM) topology analysis revealed critical points in (C6H5)2Pb, and is consistent with the literature.
 Plumbylenes occur as reactive intermediates in the formation of tetravalent
Plumbylenes occur as reactive intermediates in the formation of tetravalent plumbane
Plumbane, PbH4, is a metal hydride and group 14 hydride composed of lead and hydrogen. Plumbane is not well characterized or well known, and it is thermodynamically unstable with respect to the loss of a hydrogen atom. Derivatives of plumbane inclu ...
s (R4Pb). Although the inert pair effect suggests the divalent state should be thermodynamically more stable than the tetravalent state, in the absence of stabilizing substituents, plumbylenes are sensitive to heat and light, and tend to undergo polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer, monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are ...
and disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation states. More generally, the term can b ...
, forming elemental lead in the process.
Plumbylenes can be stabilized as monomers by the use of sterically bulky ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electro ...
s (kinetic stabilization) or heteroatom-containing substituents that can donate electron density into the vacant 6p orbital (thermodynamic stabilization).
Dimerization
 Plumbylenes are able to undergo dimerization in two ways: either through the formation of a Pb=Pb double bond to form a formal diplumbene, or through bridging halide interactions. Unhalogenated plumbylenes tend to exist in an equilibrium between the monomeric and dimeric form in solution, and, due to the low dimerization energy, as either monomers or dimers in the solid state, depending on the steric bulk of substituents. However, increasing the steric bulk of lead-bound substituents can prevent the close association of plumbylene molecules and allow the plumbylene to exist exclusively as monomers in solution or even in the solid state.
The driving force for dimerization in general arises from the Lewis amphoteric nature of plumbylenes, which possess a
Plumbylenes are able to undergo dimerization in two ways: either through the formation of a Pb=Pb double bond to form a formal diplumbene, or through bridging halide interactions. Unhalogenated plumbylenes tend to exist in an equilibrium between the monomeric and dimeric form in solution, and, due to the low dimerization energy, as either monomers or dimers in the solid state, depending on the steric bulk of substituents. However, increasing the steric bulk of lead-bound substituents can prevent the close association of plumbylene molecules and allow the plumbylene to exist exclusively as monomers in solution or even in the solid state.
The driving force for dimerization in general arises from the Lewis amphoteric nature of plumbylenes, which possess a Lewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
ic vacant 6p orbital and a weakly Lewis basic 6s lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
, which can act as electron acceptor and donor orbitals respectively. These diplumbenes possess a ''trans''-bent structure similar to that in lighter, non-carbon congeners (
These diplumbenes possess a ''trans''-bent structure similar to that in lighter, non-carbon congeners (disilene
Disilene is an inorganic compound with the chemical formula . The name ''disilene'', referring to the structure of a particular prototropic tautomer of the molecule. It is the simplest silene.
Properties and bonding
Disilene is a molecule with ...
s, digermylenes, distannylenes). The observed Pb–Pb bond lengths in diplumbenes (2.90 – 3.53 Å) have been found to typically be longer than those in tetravalent diplumbanes R3PbPbR3 (2.84 – 2.97 Å). This, together with the low computed dimerization energy (energy released from the formation of dimers from monomers) of 24 kJ mol−1 for Pb2H4, indicates weak multiple bond
In chemistry, bond order, as introduced by Linus Pauling, is defined as the difference between the number of bonds and anti-bonds.
The bond order itself is the number of electron pairs (covalent bonds) between two atoms. For example, in diatomi ...
ing. This counterintuitive result is due to the pair of 6s-6p donor-acceptor interactions representing the Pb=Pb double bond in diplumbenes being less energetically favourable compared to the overlap of spn orbitals (with a higher degree of hybridization than in diplumbenes) in the Pb–Pb single bond in diplumbanes.
In monohalogenated plumbylenes, the halogen atom on one plumbylene is able to donate a lone pair into the vacant 6p orbital of the lead atom on a separate plumbylene in a bridging mode. Monohalogenated plumbylenes have been found to generally exist as monomers in solution and dimers in the solid state, but, again, sufficiently bulky substituents on lead can sterically block this dimerization mode.
Due to decreasing dimerization energy down Group 14, while monohalogenated stannylenes and plumbylenes dimerize via the halogen-bridging mode, monohalogenated silylenes and germylenes tend to dimerize ''via'' the abovementioned multiply-bonded mode instead. In a recent study, an ''N''-heterocyclic plumbylene was shown to undergo dimerization leading to C–H activation, existing in solution in an equilibrium between the monomer and a dimer resulting from cleavage of an aryl C–H bond and formation of Pb–C and N–H bonds. DFT studies proposed that the reaction occurred via electrophilic substitution at the arene of one plumbylene by the lead atom of another, and involves concerted Pb–C and N–H bond formation instead of insertion of Pb into the C–H bond.
In a recent study, an ''N''-heterocyclic plumbylene was shown to undergo dimerization leading to C–H activation, existing in solution in an equilibrium between the monomer and a dimer resulting from cleavage of an aryl C–H bond and formation of Pb–C and N–H bonds. DFT studies proposed that the reaction occurred via electrophilic substitution at the arene of one plumbylene by the lead atom of another, and involves concerted Pb–C and N–H bond formation instead of insertion of Pb into the C–H bond.
Stabilizing intramolecular interactions with substituents bearing lone pairs
Plumbylenes may be stabilized by electron donation into the vacant orbital of the lead atom. The two common intramolecular modes are resonance from a lone pair on the atom directly attached to the lead or by coordination from aLewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
elsewhere in the molecule.
For example, Group 15 or 16 elements directly adjacent to Pb donate a lone pair in manner similar to their stabilizing effect on Fisher carbenes. Common examples of more remote electron-donors include nitrogen atoms that can lead to a six-memberd ring by bonding to the lead. Even a fluorine atom on a remote trifluoromethyl
The trifluoromethyl group is a functional group that has the formula -CF3. The naming of is group is derived from the methyl group (which has the formula -CH3), by replacing each hydrogen atom by a fluorine atom. Some common examples are trifluorom ...
group has been seen forming a coordination to lead in ,4,6-(CF3)3C6H2sub>2Pb.
Agostic interactions
Agostic interactions
In organometallic chemistry, agostic interaction refers to the interaction of a coordinatively-unsaturated transition metal with a C−H bond, when the two electrons involved in the C−H bond enter the empty d-orbital of the transition metal, ...
have also been shown to stabilize plumbylenes. DFT computations on the compounds R(CH3)2Si)CHsub>2Pb (R = Me or Ph) found that agostic interactions between bonding B–H orbitals and the vacant 6p orbital lowered the energy of the molecule by ''ca.'' 38 kcal mol−1; this was supported by X-ray crystal structures showing the favourable positioning of said B–H bonds in proximity of Pb.
Reactivity
As previously mentioned, unstabilized plumbylenes are prone to polymerization and disproportionation, and plumbylenes without bulky substituents tend to dimerize in one of two modes. Below, the reactions of stabilized plumbylenes (at least at the temperatures at which they were studied) are listed.Lewis acid-base adduct formation
Plumbylenes are Lewis acidic ''via'' the vacant 6p orbital and tend to form adducts with Lewis bases, such as trimethylamine ''N''-oxide (Me3NO), 1-azidoadamantane (AdN3), andmesityl
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenzen ...
azide (MesN3). In contrast, the reaction between stannylenes and Me3NO produces the corresponding distannoxane (from oxidation of Sn(II) to Sn(IV)) instead of the Lewis adduct, which can be attributed to tin being a period above Pb, experiencing the inert pair effect to a lesser degree and hence having a higher susceptibility to oxidation.
In the case of AdN3, the terminal N of the azidoadamantane binds to the plumbylene via a bridging mode between the Lewis acidic Pb and the Lewis basic P atom; in the case of MesN3, the azide evolves N2 to form a nitrene, which then inserts into a C-H bond of an arene substituent and coordinates to Pb as a Lewis base.

Insertion
Similar to carbenes and other Group 14 congeners, plumbylenes have been shown to undergo insertion reactions, specifically into C–X (X = Br, I) and Group 16 E–E (E = S, Se) bonds. Insertions into lead-substituent bonds can also occur.27 In the examples below, insertion is accompanied by intramolecular rearrangement to place more electron-donating heteroatoms next to the electron-deficient lead.27
Insertions into lead-substituent bonds can also occur.27 In the examples below, insertion is accompanied by intramolecular rearrangement to place more electron-donating heteroatoms next to the electron-deficient lead.27
Transmetallation
Plumbylenes are known to undergo nucleophilic substitution with organometallic reagents to form transmetallated products.28 In an unusual example, the use of TlPF6, bearing theweakly coordinating anion
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly found ...
PF6−, led to the formation of crystals of an oligonuclear lead compound with a chain structure upon work-up, highlighting the interesting reactivity of plumbylenes.28
In addition, plumbylenes can also undergo metathesis with group 13
The Group 13 network ( pl, Trzynastka, Yiddish: ''דאָס דרײַצענטל'') was a Jewish Nazi collaborationist organization in the Warsaw Ghetto during the German occupation of Poland in World War II. The rise and fall of the Group ...
E(CH3)3 (E = Al, Ga) compounds.
Plumbylenes bearing different substituents can also undergo transmetallation Transmetalation (alt. spelling: transmetallation) is a type of organometallic reaction that involves the transfer of ligands from one metal to another. It has the general form:
:M1–R + M2–R′ → M1–R′ + M2–R
where R and R′ can be, but ...
and exchange substituents, with the driving force being the relief of steric strain Van der Waals strain is strain resulting from Van der Waals repulsion when two substituents in a molecule approach each other with a distance less than the sum of their Van der Waals radii.
Van der Waals strain is also called Van der Waals repuls ...
and the low Pb-C bond dissociation energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical s ...
.

Applications
 Plumbylenes can be used as concurrent σ-donor-σ-acceptor
Plumbylenes can be used as concurrent σ-donor-σ-acceptor ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electr ...
s to metal complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
es, functioning as σ-donor via its filled 6s orbital and σ-acceptor via its empty 6p orbital.
Room temperature-stable plumbylenes have also been suggested as precursors in chemical vapour deposition (CVD) and atomic layer deposition (ALD) of lead-containing materials. Dithioplumbylenes and dialkoxyplumbylenes may be useful as precursors for preparing the semiconductor material lead sulphide Lead sulfide refers to two compounds containing lead and sulfur:
*Lead(II) sulfide
Lead is a chemical element with the symbol Pb (from the Latin ) and atomic number 82. It is a heavy metal that is denser than most common materials. Lead is s ...
and piezoelectric PZT respectively.
References
{{reflist Organolead compounds