|
Phosphorine
Phosphorine (IUPAC name: phosphinine) is a heavier element analog of pyridine, containing a phosphorus atom instead of an aza- moiety. It is also called phosphabenzene and belongs to the phosphaalkene class. It is a colorless liquid that is mainly of interest in research. Phosphorine is an air-sensitive oil but is otherwise stable when handled using air-free techniques (however, substituted derivatives can often be handled under air without risk of decomposition). In contrast, silabenzene, a related heavy-element analogue of benzene, is not only air- and moisture-sensitive but also thermally unstable without extensive steric protection. History The first phosphorine to be isolated is 2,4,6-triphenylphosphorine. It was synthesized by Gottfried Märkl in 1966 by condensation of the corresponding pyrylium salt and phosphine or its equivalent ( P(CH2OH)3 and P(SiMe3)3).''G. Märkl'', 2,4,6-Triphenylphosphabenzol iAngewandte Chemie 78, 907–908 (1966)/ref> 500px, Synthesis of T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphole
Phosphole is the organic compound with the chemical formula ; it is the phosphorus analog of pyrrole. The term phosphole also refers to substituted derivatives of the parent heterocycle. These compounds are of theoretical interest but also serve as ligands for transition metals and as precursors to more complex organophosphorus compounds. Triphosphole, , is a heterocycle with 3 phosphorus atoms. Pentaphosphole, , is a cyclic compound with 5 phosphorus atoms. Structure and bonding Unlike the related 5-membered heterocycles pyrrole, thiophene, and furan, the aromaticity of phospholes is diminished, reflecting the reluctance of phosphorus to delocalize its lone pair. The main indication of this difference is the pyramidalization of phosphorus. The absence of aromaticity is also indicated by the reactivity of phospholes. Phospholes undergo different cycloaddition reactions; coordination properties of phospholes are also well studied. Preparation The parent phosphole was first descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphole
Phosphole is the organic compound with the chemical formula ; it is the phosphorus analog of pyrrole. The term phosphole also refers to substituted derivatives of the parent heterocycle. These compounds are of theoretical interest but also serve as ligands for transition metals and as precursors to more complex organophosphorus compounds. Triphosphole, , is a heterocycle with 3 phosphorus atoms. Pentaphosphole, , is a cyclic compound with 5 phosphorus atoms. Structure and bonding Unlike the related 5-membered heterocycles pyrrole, thiophene, and furan, the aromaticity of phospholes is diminished, reflecting the reluctance of phosphorus to delocalize its lone pair. The main indication of this difference is the pyramidalization of phosphorus. The absence of aromaticity is also indicated by the reactivity of phospholes. Phospholes undergo different cycloaddition reactions; coordination properties of phospholes are also well studied. Preparation The parent phosphole was first descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society (professional association) in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 34,000 in the UK and a further 8,000 abroad. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
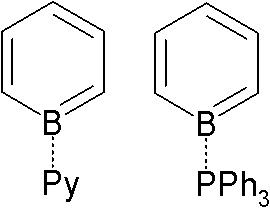
Borabenzene
Borabenzene is a hypothetical organoboron compound with the formula C5H5B. Unlike the related but highly stable benzene molecule, borabenzene would be electron-deficient. Related derivatives are the boratabenzene anions, including the parent 5H5BHsup>−. Adducts Adducts of borabenzene with Lewis bases are isolatable. Since borabenzene is unavailable, these adducts require indirect methods. 4-Silyl-1-methoxyboracyclohexadiene is used as a precursor to the borabenzene: : + → + MeOSiMe3 The pyridine adduct is structurally related to biphenyl. It is a yellow whereas biphenyl is colorless, indicating distinct electronic structures. The pyridine ligand is tightly bound: no exchange is observed with free pyridine, even at elevated temperatures. : The borabenzene-pyridine adduct behaves like a diene, not an analog of biphenyl, and will undergo Diels-Alder reactions.{{cite journal , doi=10.1021/ol061201w , title=1-Borabarrelene Derivatives via Diels−Alder Additions to Boraben ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,2′-bipyridine
2,2′-Bipyridine (bipy or bpy, pronounced ) is an organic compound with the formula C10H8N2. This colorless solid is an important isomer of the bipyridine family. It is a bidentate chelating ligand, forming complexes with many transition metals. Ruthenium and platinum complexes of bipy exhibit intense luminescence, which may have practical applications. Preparation, structure, and general properties 2,2'-Bipyridine was first prepared by decarboxylation of divalent metal derivatives of pyridine-2-carboxylate: :M(O2CC5H4N)2 → (C5H4N)2 + 2CO2 + ... It is prepared by the dehydrogenation of pyridine Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a ... using Raney nickel: :2C5H5N → (C5H4N)2 + H2 Although bipyridine is often drawn with its nitrogen atoms in ''cis'' conformation, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acylation
In chemistry, acylation (or alkanoylation) is the chemical reaction in which an acyl group () is added to a compound. The compound providing the acyl group is called the acylating agent. Because they form a strong electrophile when treated with some metal catalysts, acyl halides are commonly used as acylating agents. For example, Friedel–Crafts acylation uses acetyl chloride (ethanoyl chloride or ) as the agent and aluminum chloride () as a catalyst to add an ethanoyl ( acetyl) group to benzene: The mechanism of this reaction is electrophilic aromatic substitution. Acyl halides and acid anhydrides of carboxylic acids are also commonly used acylating agents. In some cases, active esters exhibit comparable reactivity. All react with amines to form amides and with alcohols to form esters by nucleophilic acyl substitution. Acylation can be used to prevent rearrangement reactions that would normally occur in alkylation. To do this an acylation reaction is performed, then the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bromination
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens (F2, Cl2, Br2, I2). Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride. Organic chemistry Several pathways exist for the halogenation of organic compounds, including free radical halogenation, ketone halogenation, electrophilic halogenation, and halogen addition reaction. The nature of the substrate determines the pathway. The facility of halogenation is influenced by the halogen. Fluorine and chlorine are more electrophilic and are m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyllithium
Methyllithium is the simplest organolithium reagent with the empirical formula CH3Li. This s-block organometallic compound adopts an oligomeric structure both in solution and in the solid state. This highly reactive compound, invariably used in solution with an ether as the solvent, is a reagent in organic synthesis as well as organometallic chemistry. Operations involving methyllithium require anhydrous conditions, because the compound is highly reactive toward water. Oxygen and carbon dioxide are also incompatible with MeLi. Methyllithium is usually not prepared, but purchased as a solution in various ethers. Synthesis In the direct synthesis, methyl bromide is treated with a suspension of lithium in diethyl ether. :2 Li + MeBr → LiMe + LiBr The lithium bromide forms a complex with the methyllithium. Most commercially available methyllithium consists of this complex. "Halide-free" methyllithium is prepared from methyl chloride. Lithium chloride precipitates from the diethyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Lengths Of Group 15 Heterobenzenes And Benzene
Bond or bonds may refer to: Common meanings * Bond (finance), a type of debt security * Bail bond, a commercial third-party guarantor of surety bonds in the United States * Chemical bond, the attraction of atoms, ions or molecules to form chemical compounds People * Bond (surname) * Bonds (surname) * Mr. Bond (musician), Austrian rapper Arts and entertainment * James Bond, a series of works about the eponymous fictional character * James Bond (literary character), a British secret agent in a series of novels and films * Bond (band), an Australian/British string quartet ** '' Bond: Video Clip Collection'', a video collection from the band * Bond (Canadian band), a Canadian rock band in the 1970s * ''The Bond'' (2007 book), an American autobiography written by The Three Doctors * ''The Bond'', a 1918 film by Charlie Chaplin supporting Liberty bonds * Bond International Casino, a former music venue in New York City Places Antarctica * Bond Glacier, at the head of Vincennes Ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Picometer
The picometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: pm) or picometer (American spelling) is a unit of length in the International System of Units (SI), equal to , or one trillionth of a metre, which is the SI base unit of length. The picometre is one thousand femtometres, one thousandth of a nanometre ( nm), one millionth of a micrometre (also known as a micron), one billionth of a millimetre, and one trillionth of a metre. The symbol μμ was once used for it. It is also one hundredth of an ångström, an internationally known (but non-SI) unit of length. Use The picometre's length is of an order so small that its application is almost entirely confined to particle physics, quantum physics, chemistry and acoustics. Atoms are between 62 and 520 pm in diameter, and the typical length of a carbon–carbon single bond is 154 pm. Smaller units still may be used to describe smaller particles (some of which are t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule. Explanation Bond length is related to bond order: when more electrons participate in bond formation the bond is shorter. Bond length is also inversely related to bond strength and the bond dissociation energy: all other factors being equal, a stronger bond will be shorter. In a bond between two identical atoms, half the bond distance is equal to the covalent radius. Bond lengths are measured in the solid phase by means of X-ray diffraction, or approximated in the gas phase by microwave spectroscopy. A bond between a given pair of atoms may vary between different molecules. For example, the carbon to hydrogen bonds in methane are different from those in methyl chloride. It is however possible to make generalizations when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |