|
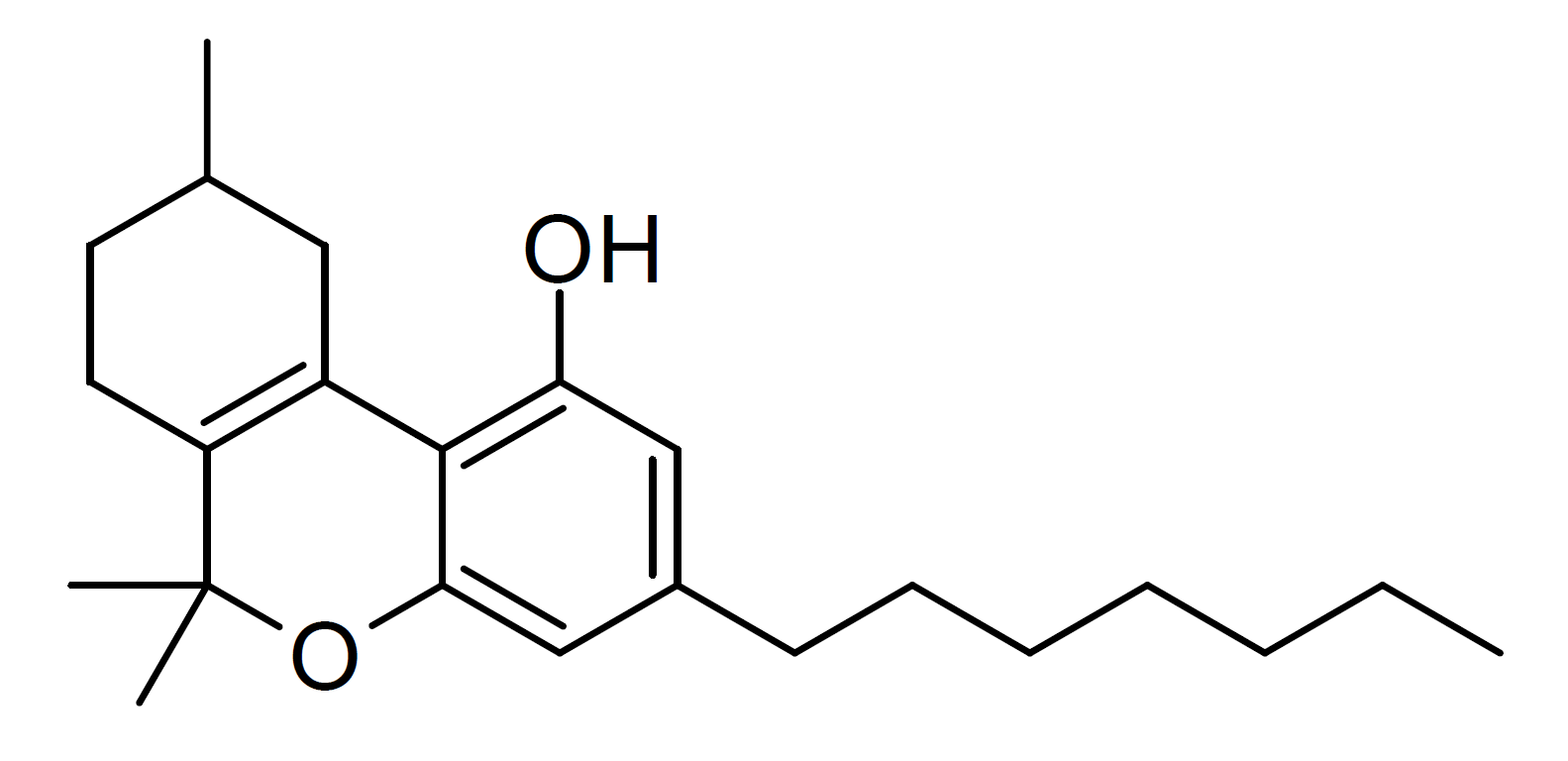
Parahexyl
Parahexyl (Synhexyl, n-hexyl-Δ3-THC, (C6)-Δ6a(10a)-THC) is a synthetic homologue of THC which was invented in 1941 during attempts to elucidate the structure of Δ9-THC, one of the active components of cannabis. Parahexyl is similar in both structure and activity to THC, differing only in the position of one double bond and the lengthening of the chain by one CH2 group to . Parahexyl produces effects typical of other cannabinoid receptor agonists in animals. It has a somewhat higher oral bioavailability than THC itself but is otherwise very similar. Presumably, it acts as a CB1 agonist in the same way as THC, but as there has been no research published using parahexyl since the discovery of the CB1 receptor, this has not been definitively confirmed. Parahexyl was occasionally used as an anxiolytic in the mid-20th century, the dosage ranging from 5 mg to 90 mg. Parahexyl was made illegal under UN convention in 1982 on the basis of its structural similarity and sim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrocannabinol
Tetrahydrocannabinol (THC) is the principal psychoactive constituent of cannabis and one of at least 113 total cannabinoids identified on the plant. Although the chemical formula for THC (C21H30O2) describes multiple isomers, the term ''THC'' usually refers to the Delta-9-THC isomer with chemical name (−)-''trans''-Δ9-tetrahydrocannabinol. THC is a lipid found in cannabis and, like most pharmacologically active secondary metabolites of plants, it is assumed to be involved in the plant's evolutionary adaptation, putatively against insect predation, ultraviolet light, and environmental stress. THC was first discovered and isolated by Israeli chemist Raphael Mechoulam in Israel in 1964. It was found that, when smoked, THC is absorbed into the bloodstream and travels to the brain, attaching itself to endocannabinoid receptors located in the cerebral cortex, cerebellum, and basal ganglia. These are the parts of the brain responsible for thinking, memory, pleasure, coordi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrocannabiphorol
Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a CB1 and CB2 agonist which was known as a synthetic homologue of THC, but for the first time in 2019 was isolated as a natural product in trace amounts from ''Cannabis sativa''. It is structurally similar to Δ9-THC, the main active component of cannabis, but with the pentyl side chain extended to heptyl. Since it has a longer side chain, its cannabinoid effects are "far higher than Δ9-THC itself." Tetrahydrocannabiphorol has a reported binding affinity approximately 33 times that of Delta-9-THC. Isomers Delta-3-THCP ] The Δ3/Δ6a(10a) isomer Δ3-THCP was synthesised in 1941, and was found to have around the same potency as Delta-3-Tetrahydrocannabinol, Δ3-THC, unlike the hexyl homologue parahexyl which was significantly stronger. Delta-8-THCP The Δ8 isomer is also known as a synthetic cannabinoid under the code name JWH-091, It's unconfirmed whether or not Delta-8-THCP is found naturally in cannabis plan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrocannabihexol
Tetrahydrocannabihexol (Δ9-THCH, Δ9-Parahexyl, n-Hexyl-Δ9-THC) is a phytocannabinoid, the hexyl homologue of tetrahydrocannabinol (THC) which was first isolated from ''Cannabis'' plant material in 2020 along with the corresponding hexyl homologue of cannabidiol, though it had been known for several decades prior to this as an isomer of the synthetic cannabinoid parahexyl. Another isomer Δ8-THCH is also known as a synthetic cannabinoid under the code number JWH-124, though it is unclear whether this occurs naturally in ''Cannabis'', but likely is due to Delta-8-THC itself being a degraded form of Delta-9-THC. ] ] See also * Cannabidiphorol * Tetrahydrocannabiorcol * Tetrahydrocannabivarin * Tetrahydrocannabutol * Tetrahydrocannabiphorol Tetrahydrocannabiphorol (THCP) is a potent phytocannabinoid, a CB1 and CB2 agonist which was known as a synthetic homologue of THC, but for the first time in 2019 was isolated as a natural product in trace amounts from ''Cannabis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Convention On Psychotropic Substances
The Convention on Psychotropic Substances of 1971 is a United Nations treaty designed to control psychoactive drugs such as amphetamine-type stimulants, barbiturates, benzodiazepines, and psychedelics signed in Vienna, Austria on 21 February 1971. The Single Convention on Narcotic Drugs of 1961 did not ban the many newly discovered psychotropics, since its scope was limited to drugs with cannabis, coca and opium-like effects. During the 1960s such drugs became widely available, and government authorities opposed this for numerous reasons, arguing that along with negative health effects, drug use led to lowered moral standards. The Convention, which contains import and export restrictions and other rules aimed at limiting drug use to scientific and medical purposes, came into force on 16 August 1976. As of 2013, 183 member states are Parties to the treaty. Many laws have been passed to implement the Convention, including the Canadian Controlled Drugs and Substances Act, th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homology (chemistry)
In chemistry, homology is the appearance of homologues. A homologue (also spelled as homolog) is a compound belonging to a series of compounds differing from each other by a repeating unit, such as a methylene bridge −−, a peptide residue, etc. A homolog is a special case of an analog. Examples are alkanes and compounds with alkyl side chains of different length (the repeating unit being a methylene group -CH2-). Periodic table On the periodic table, homologous elements share many electrochemical properties and appear in the same group (column) of the table. For example, all noble gases are colorless, monatomic gases with very low reactivity. These similarities are due to similar structure in their outer shells of valence electrons. Mendeleev used the prefix eka- for an unknown element below a known one in the same group. See also * Homologous series * Analog * Congener * Structure–activity relationship The structure–activity relationship (SAR) is th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrocannabivarin
Tetrahydrocannabivarin (THCV, THV, O-4394, GWP42004) is a homologue of tetrahydrocannabinol (THC) having a propyl (3-carbon) side chain instead of a pentyl (5-carbon) group on the molecule, which makes it produce very different effects from THC. Natural occurrence THCV is prevalent in certain central Asian and southern African strains of ''Cannabis''. Chemistry Similar to THC, THCV has 7 possible double bond isomers and 30 stereoisomers (see: Tetrahydrocannabinol#Isomerism). The alternative isomer Δ8-THCV is known as a synthetic compound with a code number of O-4395, but it is not known to have been isolated from ''Cannabis'' plant material. ] Description Plants with elevated levels of propyl cannabinoids (including THCV) have been found in populations of ''Cannabis sativa'' L. ssp. ''indica'' (= ''Cannabis indica'' Lam.) from China, India, Nepal, Thailand, Afghanistan, and Pakistan, as well as southern and western Africa. THCV levels up to 53.7% of total cannabinoi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrocannabutol
Δ9-Tetrahydrocannabutol (tetrahydrocannabinol-C4, THC-C4, Δ9-THCB, (C4)-Δ9-THC, butyl-THC) is a phytocannabinoid found in cannabis that is a homologue of tetrahydrocannabinol (THC), the main active component of Cannabis. Structurally, they are only different by the pentyl side chain being replaced by a butyl side chain. Pharmacology Δ9-THCB, showed an affinity for the human CB1 (''K''i = 15 nM) and CB2 receptors (''K''i = 51 nM) comparable to that of Δ9-THC. The formalin test in vivo was performed on Δ9-THCB in order to reveal possible analgesic and anti-inflammatory properties. The tetrad test in mice showed a partial agonistic activity of Δ9-THCB toward the CB1 receptor. THCB has rarely been isolated from cannabis samples, but appears to be less commonly present than THC or THCV. It is metabolized in a similar manner to THC. In an analysis by the University of Rhode Island on phytocannabinoids it was found that THC-Butyl had the highest 3C-like protease inhibitor a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Delta-3-Tetrahydrocannabinol
Delta-3-Tetrahydrocannabinol (Delta-3-THC, Δ3-THC, Δ6a(10a)-THC, EA-1477) is a synthetic isomer of tetrahydrocannabinol, developed during the original research in the 1940s to develop synthetic routes to the natural products Δ8-THC and Δ9-THC found in the cannabis plant. While the normal ''trans'' configuration of THC is in this case flattened by the double bond, it still has two enantiomers as the 9-methyl group can exist in an (R) or (S) conformation. The (S) enantiomer has similar effects to Δ9-THC though with several times lower potency, while the (R) enantiomer is many times less active or inactive, depending on the assay used. See also * 7,8-Dihydrocannabinol * Cannabitriol * Delta-4-Tetrahydrocannabinol * Delta-7-Tetrahydrocannabinol * Delta-10-Tetrahydrocannabinol * Hexahydrocannabinol * JWH-138 * Parahexyl Parahexyl (Synhexyl, n-hexyl-Δ3-THC, (C6)-Δ6a(10a)-THC) is a synthetic homologue of THC which was invented in 1941 during attempts to elucidate the stru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dibenzopyran And Monoterpenoid Numbering Of Tetrahydrocannabinol
Xanthene (9''H''-xanthene, 10''H''-9-oxaanthracene) is the organic compound with the formula CH2 6H4sub>2O. It is a yellow solid that is soluble in common organic solvents. Xanthene itself is an obscure compound, but many of its derivatives are useful dyes. Xanthene dyes Dyes that contain a xanthene core include fluorescein, eosins, and rhodamines. Xanthene dyes tend to be fluorescent, yellow to pink to bluish red, brilliant dyes. Many xanthene dyes can be prepared by condensation of derivates of phthalic anhydride with derivates of resorcinol or 3-aminophenol. Further reading * See also * Xanthone * Xanthydrol Xanthydrol is an organic chemical compound. Its formula is C13 H10 O2. Its total molecular weight is 198.221 g/ mol. Xanthydrol is used to test the levels of urea in the bloodstream. Synthesis Xanthydrol can be produced by the reduction of xan ... References {{reflist Fungicides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
United Nations
The United Nations (UN) is an intergovernmental organization whose stated purposes are to maintain international peace and security, develop friendly relations among nations, achieve international cooperation, and be a centre for harmonizing the actions of nations. It is the world's largest and most familiar international organization. The UN is headquartered on international territory in New York City, and has other main offices in Geneva, Nairobi, Vienna, and The Hague (home to the International Court of Justice). The UN was established after World War II with the aim of preventing future world wars, succeeding the League of Nations, which was characterized as ineffective. On 25 April 1945, 50 governments met in San Francisco for a conference and started drafting the UN Charter, which was adopted on 25 June 1945 and took effect on 24 October 1945, when the UN began operations. Pursuant to the Charter, the organization's objectives include maintaining internationa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anxiolytic
An anxiolytic (; also antipanic or antianxiety agent) is a medication or other intervention that reduces anxiety. This effect is in contrast to anxiogenic agents which increase anxiety. Anxiolytic medications are used for the treatment of anxiety disorders and their related psychological and physical symptoms. Nature of anxiety Anxiety is a naturally-occurring emotion and an innate response of the body to the environmental stimuli. Mild to moderate anxiety would increase level of performance. However, when anxiety levels exceed the tolerability of a person, anxiety disorders may occur. People with anxiety disorders can exhibit fear responses such as defensive behaviors, high levels of alertness and negative emotions, without external stimuli which induce anxiety within an individual. Those with anxiety disorders are also often found to have concurrent psychological disorders, most commonly depression. Anxiety disorders are divided into 6 types in clinical recognition. They are a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |