|
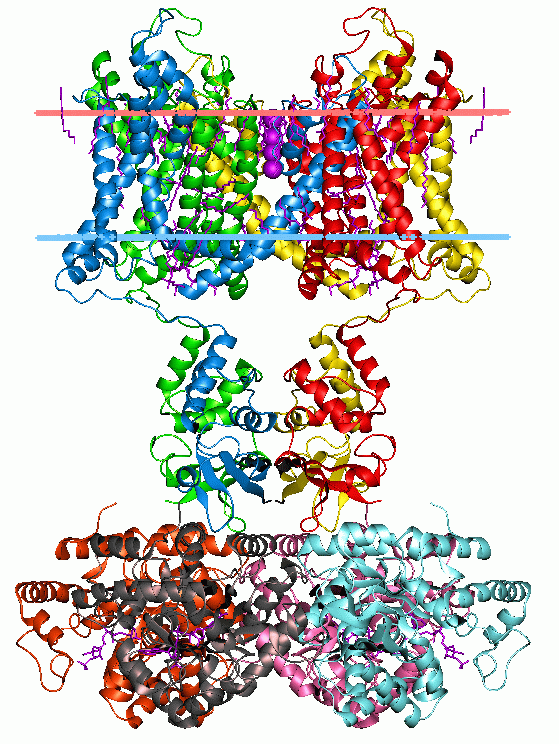
PTS Mannose-Fructose-Sorbose Family
The PTS Mannose-Fructose-Sorbose (Man) FamilyTC# 4.A.6 is a group of multicomponent PTS systems that are involved in sugar uptake in bacteria. This transport process is dependent on several cytoplasmic phosphoryl transfer proteins - Enzyme I (I), HPr, Enzyme IIA (IIA), and Enzyme IIB (IIB) as well as the integral membrane sugar permease complex (IICD). It is not part of the PTS-AG or PTS-GFL superfamilies. Distinguishing characteristics from other PTS porters The Man Family is unique in several respects among other PTS porter families: # It is the only PTS family in which members possess a IID protein; # It is the only PTS family in which the IIB constituent is phosphorylated on a histidyl rather than a cysteyl residue; # Its porter members usually exhibit broad specificity for a range of sugars, rather than being specific for just one or a few sugars. The mannose porter of ''Escherichia coli'', for example, can transport and phosphorylate glucose, mannose, fructose, gluc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphotransferase System
PEP group translocation, also known as the phosphotransferase system or PTS, is a distinct method used by bacteria for sugar uptake where the source of energy is from phosphoenolpyruvate (PEP). It is known to be a multicomponent system that always involves enzymes of the plasma membrane and those in the cytoplasm. The PTS system uses active transport. After the translocation across the membrane, the metabolites transported are modified. The system was discovered by Saul Roseman in 1964. The bacterial phosphoenolpyruvate:sugar phosphotransferase system (PTS) transports and phosphorylates its sugar substrates in a single energy-coupled step. This transport process is dependent on several cytoplasmic phosphoryl transfer proteins - Enzyme I (I), HPr, Enzyme IIA (IIA), and Enzyme IIB (IIB)) as well as the integral membrane sugar permease (IIC).The PTS Enzyme II complexes are derived from independently evolving 4 PTS Enzyme II complex superfamilies, that include the (1) Glucose (Glc),(2) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PTS-AG Superfamily
Permease of phosphotransferase system (or PTS-AG superfamily according to TCDB) is a superfamily of phosphotransferase enzymes that facilitate the transport of L-ascorbate (A) and galactitol Galactitol (dulcitol) is a sugar alcohol, the reduction product of galactose. It has a slightly sweet taste. In people with galactokinase deficiency, a form of galactosemia, excess dulcitol forms in the lens of the eye leading to cataracts. Gala ... (G). Classification has been established through phylogenic analysis and bioinformatics. The bacterial phosphoenolpyruvate:sugar phosphotransferase system (PTS) transports and phosphorylates its sugar substrates in a single energy-coupled step. This transport process is dependent on several cytoplasmic phosphoryl transfer proteins - Enzyme I (I), HPr, Enzyme IIA (IIA), and Enzyme IIB (IIB)) as well as the integral membrane sugar permease (IIC). The PTS Enzyme II complexes are derived from independently evolving 4 PTS Enzyme II complex superf ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PTS-GFL Superfamily
The phosphotransferases system (PTS-GFL) superfamily is a Protein superfamily, superfamily of Phosphotransferase, phosphotransferase enzymes that facilitate the transport of glucose, glucitol (G), fructose (F) and lactose (L). Classification has been established through phylogenic analysis and bioinformatics. The bacterial phosphoenolpyruvate:sugar phosphotransferase system (PTS) transports and phosphorylates its sugar substrates in a single energy-coupled step. This transport process is dependent on several cytoplasmic phosphoryl transfer proteins - Enzyme I (I), HPr, Enzyme IIA (IIA), and Enzyme IIB (IIB)) as well as the integral membrane sugar permease (IIC). The PTS Enzyme II complexes are derived from independently evolving 4 PTS Enzyme II complex superfamilies, that include the (1) Glucose (Glc),(2) Mannose (Man), (3) Ascorbate-Galactitol (Asc-Gat) and (4) Dihydroxyacetone (Dha) superfamilies. The four families that make up the PTS-GFL superfamily include: 4.A.1– The PTS ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histidine
Histidine (symbol His or H) is an essential amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), a carboxylic acid group (which is in the deprotonated –COO− form under biological conditions), and an imidazole side chain (which is partially protonated), classifying it as a positively charged amino acid at physiological pH. Initially thought essential only for infants, it has now been shown in longer-term studies to be essential for adults also. It is encoded by the codons CAU and CAC. Histidine was first isolated by Albrecht Kossel and Sven Gustaf Hedin in 1896. It is also a precursor to histamine, a vital inflammatory agent in immune responses. The acyl radical is histidyl. Properties of the imidazole side chain The conjugate acid (protonated form) of the imidazole side chain in histidine has a p''K''a of approximately 6.0. Thus, below a pH of 6, the imidazole ring ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of designating chi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Escherichia Coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escherichia'' that is commonly found in the lower intestine of warm-blooded organisms. Most ''E. coli'' strains are harmless, but some serotypes ( EPEC, ETEC etc.) can cause serious food poisoning in their hosts, and are occasionally responsible for food contamination incidents that prompt product recalls. Most strains do not cause disease in humans and are part of the normal microbiota of the gut; such strains are harmless or even beneficial to humans (although these strains tend to be less studied than the pathogenic ones). For example, some strains of ''E. coli'' benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by pathogenic bacteria. These mutually beneficial relationships between ''E. col ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoenolpyruvate
Phosphoenolpyruvate (2-phosphoenolpyruvate, PEP) is the ester derived from the enol of pyruvate and phosphate. It exists as an anion. PEP is an important intermediate in biochemistry. It has the highest-energy phosphate bond found (−61.9 kJ/mol) in organisms, and is involved in glycolysis and gluconeogenesis. In plants, it is also involved in the biosynthesis of various aromatic compounds, and in carbon fixation; in bacteria, it is also used as the source of energy for the phosphotransferase system. In glycolysis PEP is formed by the action of the enzyme enolase on 2-phosphoglyceric acid. Metabolism of PEP to pyruvic acid by pyruvate kinase (PK) generates adenosine triphosphate (ATP) via substrate-level phosphorylation. ATP is one of the major currencies of chemical energy within cells. In gluconeogenesis PEP is formed from the decarboxylation of oxaloacetate and hydrolysis of one guanosine triphosphate molecule. This reaction is catalyzed by the enzyme pho ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Proteins
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (transmembrane) or associate with one or the other side of a membrane ( integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane. Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs. Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct conformation of the protein in isolation from its native environment. Function Membrane proteins perform a variety of functions vital to the surv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |