|
PSRK
PSRK (short for Predictive Soave–Redlich–Kwong) is an estimation method for the calculation of phase equilibria of mixtures of chemical components. The original goal for the development of this method was to enable the estimation of properties of mixtures containing supercritical components. This class of substances cannot be predicted with established models, for example UNIFAC. Principle PSRK is a group-contribution equation of state. This is a class of prediction methods that combines equations of state (mostly cubic) with activity coefficient models based on group contributions, such as UNIFAC. The activity coefficient model is used to adapt the equation-of-state parameters for mixtures by a so-called mixing rule. The use of an equation of state introduces all thermodynamic relations defined for equations of state into the PRSK model. This allows the calculation of densities, enthalpies, heat capacities, and other properties. Equations As stated previously, the PSR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PSRK VLE Prediction Cyclohexane And Carbon Dioxide
PSRK (short for Predictive Soave–Redlich–Kwong) is an estimation method for the calculation of phase equilibria of mixtures of chemical components. The original goal for the development of this method was to enable the estimation of properties of mixtures containing supercritical components. This class of substances cannot be predicted with established models, for example UNIFAC. Principle PSRK is a group-contribution equation of state. This is a class of prediction methods that combines equations of state (mostly cubic) with activity coefficient models based on Group-contribution method, group contributions, such as UNIFAC. The activity coefficient model is used to adapt the equation-of-state parameters for mixtures by a so-called mixing rule. The use of an equation of state introduces all thermodynamic relations defined for equations of state into the PRSK model. This allows the calculation of Density, densities, Enthalpy, enthalpies, Heat capacity, heat capacities, and othe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
UNIFAC Consortium
The UNIFAC Consortium was founded at the Carl von Ossietzky University of Oldenburg at the chair of industrial chemistry of Prof. Gmehling to invite private companies to support the further development of the group-contribution methods UNIFAC and its successor modified UNIFAC (Dortmund). Both models are used for the prediction of thermodynamic properties, especially the estimation of phase equilibria. The UNIFAC consortium is a successful example of private sponsorship of a public university in Germany. History The consortium was founded in 1997 when the public financing of the further development of the models became unlikely. The models UNIFAC and mod. UNIFAC (Dortmund) have already been used widely in software for the simulation and synthesis of chemical processes. Many companies doing process development in the field of chemical engineering had announced their support for a new way to subsidize the further development. This is facilitated through the support of over 40 com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Critical Pressure
In thermodynamics, a critical point (or critical state) is the end point of a phase equilibrium curve. The most prominent example is the liquid–vapor critical point, the end point of the pressure–temperature curve that designates conditions under which a liquid and its vapor can coexist. At higher temperatures, the gas cannot be liquefied by pressure alone. At the critical point, defined by a ''critical temperature'' ''T''c and a ''critical pressure'' ''p''c, phase boundaries vanish. Other examples include the liquid–liquid critical points in mixtures, and the ferromagnet–paramagnet transition (Curie temperature) in the absence of an external magnetic field. Liquid–vapor critical point Overview For simplicity and clarity, the generic notion of ''critical point'' is best introduced by discussing a specific example, the vapor–liquid critical point. This was the first critical point to be discovered, and it is still the best known and most studied one. The figu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dortmund Data Bank
The Dortmund Data Bank (short DDB) is a factual data bank for thermodynamic and thermophysical data. Its main usage is the data supply for process simulation where experimental data are the basis for the design, analysis, synthesis, and optimization of chemical processes. The DDB is used for fitting parameters for thermodynamic models like NRTL or UNIQUAC and for many different equations describing pure component properties, e.g., the Antoine equation for vapor pressures. The DDB is also used for the development and revision of predictive methods like UNIFAC and PSRK. Contents Mixture properties * Phase equilibria data ( vapor–liquid, liquid–liquid, solid–liquid), data on azeotropy and zeotropy * Mixing enthalpies * Gas solubilities * Activity coefficients at infinite dilution * Heat capacities and excess heat capacities * Volumes, densities, and excess volumes (volume effect of mixing) * Salt solubilities * Octanol-water partition coefficients * Critical d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
VTPR
VTPR (short for Volume-Translated Peng–Robinson) is an estimation method for the calculation of phase equilibria of mixtures of chemical components. The original goal for the development of this method was to enable the estimation of properties of mixtures which contain supercritical components. These class of substances couldn't be predicted with established models like UNIFAC. Principle VTPR is a group contribution equation of state. This is class of prediction methods combine equations of state (mostly cubic) with activity coefficient models based on group contributions like UNIFAC. The activity coefficient model is used to adapt the equation of state parameters for mixtures by a so-called mixing rule. The usage of an equation of state introduces all thermodynamic relations defined for equations of state into the VTPR model. This allows the calculation of densities, enthalpies, heat capacities, and more. Equations VTPR is based on a combination of the Peng–Robinson e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
UNIFAC
In statistical thermodynamics, the UNIFAC method ( UNIQUAC Functional-group Activity Coefficients)Aage Fredenslund, Russell L. Jones and John M. Prausnitz, "Group-Contribution Estimation of Activity Coefficients in Nonideal Liquid Mixtures", ''AIChE Journal'', vol. 21 (1975), p. 1086 is a semi-empirical system for the prediction of non-electrolyte activity in non- ideal mixtures. UNIFAC uses the functional groups present on the molecules that make up the liquid mixture to calculate activity coefficients. By using interactions for each of the functional groups present on the molecules, as well as some binary interaction coefficients, the activity of each of the solutions can be calculated. This information can be used to obtain information on liquid equilibria, which is useful in many thermodynamic calculations, such as chemical reactor design, and distillation calculations. The UNIFAC model was first published in 1975 by Fredenslund, Jones and John Prausnitz, a group of chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
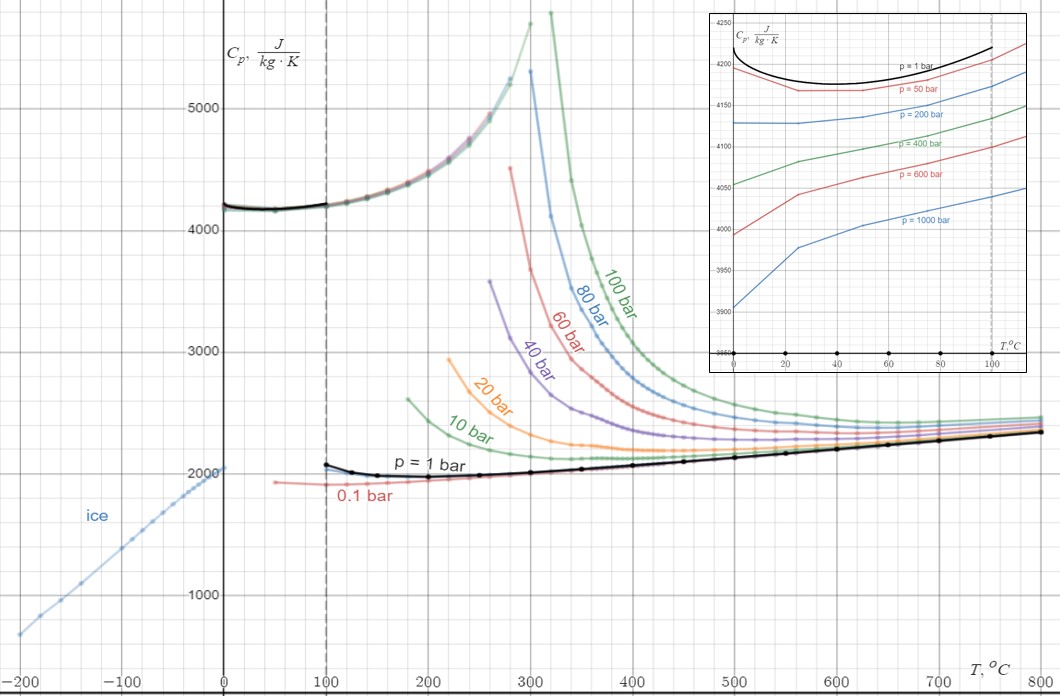
Heat Capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K). Heat capacity is an extensive property. The corresponding intensive property is the specific heat capacity, found by dividing the heat capacity of an object by its mass. Dividing the heat capacity by the amount of substance in moles yields its molar heat capacity. The volumetric heat capacity measures the heat capacity per volume. In architecture and civil engineering, the heat capacity of a building is often referred to as its thermal mass. Definition Basic definition The heat capacity of an object, denoted by C, is the limit : C = \lim_\frac, where \Delta Q is the amount of heat that must be added to the object (of mass ''M'') in order to raise its temperature by \Delta T. The value of this parameter usually varies considerably depending on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equation Of State
In physics, chemistry, and thermodynamics, an equation of state is a thermodynamic equation relating state variables, which describe the state of matter under a given set of physical conditions, such as pressure, volume, temperature, or internal energy. Most modern equations of state are formulated in the Helmholtz free energy. Equations of state are useful in describing the properties of pure substances and mixtures in liquids, gases, and solid states as well as the state of matter in the interior of stars. Overview At present, there is no single equation of state that accurately predicts the properties of all substances under all conditions. An example of an equation of state correlates densities of gases and liquids to temperatures and pressures, known as the ideal gas law, which is roughly accurate for weakly polar gases at low pressures and moderate temperatures. This equation becomes increasingly inaccurate at higher pressures and lower temperatures, and fails to pred ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acentric Factor
The acentric factor is a conceptual number introduced by Kenneth Pitzer in 1955, proven to be useful in the description of fluids. It has become a standard for the phase characterization of single & pure components, along with other state description parameters such as molecular weight, critical temperature, critical pressure, and critical volume (or critical compressibility). Pitzer defined from the relationship :\omega = - \log_ (p^_r) - 1, T_r = 0.7 where p^_r = \frac is the reduced saturation vapor pressure and T_r = \frac is the reduced temperature. The acentric factor is said to be a measure of the non-sphericity (centricity) of molecules. As it increases, the vapor curve is "pulled" down, resulting in higher boiling points. For many monatomic fluids, p_r^ T_r = 0.7 is close to 0.1, which leads to \omega \to 0. In many cases, T_r = 0.7 lies above the boiling temperature of liquids at atmosphere pressure. Values of can be determined for any fluid from accurate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Critical Temperature
Critical or Critically may refer to: *Critical, or critical but stable, medical states **Critical, or intensive care medicine * Critical juncture, a discontinuous change studied in the social sciences. * Critical Software, a company specializing in mission and business critical information systems * Critical theory, a school of thought that critiques society and culture by applying knowledge from the social sciences and the humanities * Critically endangered, a risk status for wild species *Criticality (status), the condition of sustaining a nuclear chain reaction Art, entertainment, and media * ''Critical'' (novel), a medical thriller written by Robin Cook * ''Critical'' (TV series), a Sky 1 TV series * "Critical" (''Person of Interest''), an episode of the American television drama series ''Person of Interest'' *"Critical", a 1999 single by Zion I People *Cr1TiKaL (born 1994), an American YouTuber and Twitch streamer See also *Critic *Criticality (other) *Critical Con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equation Of State
In physics, chemistry, and thermodynamics, an equation of state is a thermodynamic equation relating state variables, which describe the state of matter under a given set of physical conditions, such as pressure, volume, temperature, or internal energy. Most modern equations of state are formulated in the Helmholtz free energy. Equations of state are useful in describing the properties of pure substances and mixtures in liquids, gases, and solid states as well as the state of matter in the interior of stars. Overview At present, there is no single equation of state that accurately predicts the properties of all substances under all conditions. An example of an equation of state correlates densities of gases and liquids to temperatures and pressures, known as the ideal gas law, which is roughly accurate for weakly polar gases at low pressures and moderate temperatures. This equation becomes increasingly inaccurate at higher pressures and lower temperatures, and fails to pred ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant pressure, which is conveniently provided by the large ambient atmosphere. The pressure–volume term expresses the work required to establish the system's physical dimensions, i.e. to make room for it by displacing its surroundings. The pressure-volume term is very small for solids and liquids at common conditions, and fairly small for gases. Therefore, enthalpy is a stand-in for energy in chemical systems; bond, lattice, solvation and other "energies" in chemistry are actually enthalpy differences. As a state function, enthalpy depends only on the final configuration of internal energy, pressure, and volume, not on the path taken to achieve it. In the International System of Units (SI), the unit of measurement for enthalpy is the joule ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |