|
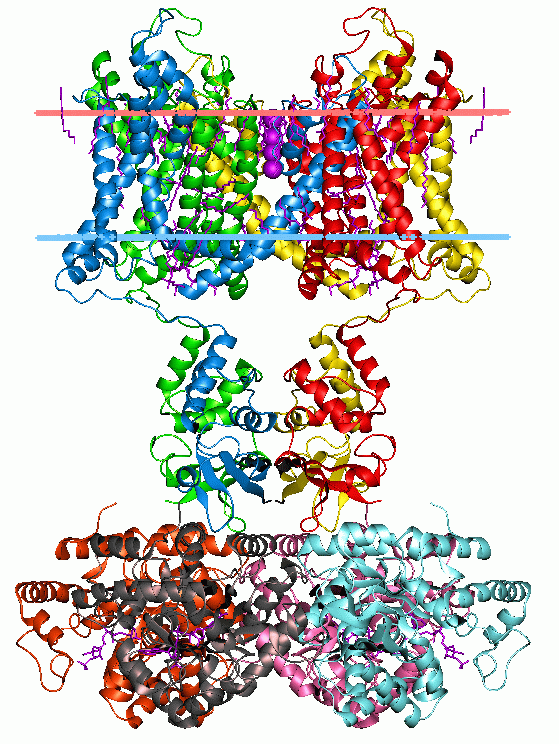
P2Y Receptor
P2Y receptors are a family of purinergic G protein-coupled receptors, stimulated by nucleotides such as adenosine triphosphate, adenosine diphosphate, uridine triphosphate, uridine diphosphate and UDP-glucose.To date, 8 P2Y receptors have been cloned in humans: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14. P2Y receptors are present in almost all human tissues where they exert various biological functions based on their G-protein coupling. P2Y receptors mediate responses including vasodilation, blood clotting, and immune response. Due to their ubiquity and variety in function, they are a common biological target in pharmacological development. Structure P2Y receptors are membrane proteins belonging to the class A family of G protein-coupled receptors (GPCRs). P2Y receptor proteins display large-scale structural domains typical of GPCRs, consisting of seven hydrophobic transmembrane helices connected by three short extracellular loops and three variably siz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Purinergic Receptor
Purinergic receptors, also known as purinoceptors, are a family of plasma membrane molecules that are found in almost all mammalian tissues. Within the field of purinergic signalling, these receptors have been implicated in learning and memory, locomotor and feeding behavior, and sleep. More specifically, they are involved in several cellular functions, including proliferation and migration of neural stem cells, vascular reactivity, apoptosis and cytokine secretion. These functions have not been well characterized and the effect of the extracellular microenvironment on their function is also poorly understood. The term ''purinergic receptor'' was originally introduced to illustrate specific classes of membrane receptors that mediate relaxation of gut smooth muscle as a response to the release of ATP (P2 receptors) or adenosine (P1 receptors). P2 receptors have further been divided into five subclasses: P2X, P2Y, P2Z, P2U, and P2T. To distinguish P2 receptors further, the subclass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Human Tissue
In biology, tissue is a biological organizational level between cells and a complete organ. A tissue is an ensemble of similar cells and their extracellular matrix from the same origin that together carry out a specific function. Organs are then formed by the functional grouping together of multiple tissues. The English word "tissue" derives from the French word "tissu", the past participle of the verb tisser, "to weave". The study of tissues is known as histology or, in connection with disease, as histopathology. Xavier Bichat is considered as the "Father of Histology". Plant histology is studied in both plant anatomy and physiology. The classical tools for studying tissues are the paraffin block in which tissue is embedded and then sectioned, the histological stain, and the optical microscope. Developments in electron microscopy, immunofluorescence, and the use of frozen tissue-sections have enhanced the detail that can be observed in tissues. With these tools, the clas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfide
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. ''Persulfide'' usually refers to compounds. In inorganic chemistry disulfide usually refers to the corresponding anion (−S−S−). Organic disulfides Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organo sulfur chemistry are symmetrical disulfides. Unsymmetrical disulfides (also called heterodisulfides) are compounds of the formula . They are less common in organic chemistry, but most disulfides in nature are unsymmetrical. Properties The disulfide bonds are strong, with a typical bond dissociation energy of 60 kcal/mol (251&nbs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of designating chi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterodimers
In biochemistry, a protein dimer is a macromolecular complex formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", '' di-'' + ''-mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins. A protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and heterodimers with several ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homodimers
In biochemistry, a protein dimer is a macromolecular complex formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", '' di-'' + '' -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins. A protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and heterodimers with several typ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Helix
A transmembrane domain (TMD) is a membrane-spanning protein domain. TMDs generally adopt an alpha helix topological conformation, although some TMDs such as those in porins can adopt a different conformation. Because the interior of the lipid bilayer is hydrophobic, the amino acid residues in TMDs are often hydrophobic, although proteins such as membrane pumps and ion channels can contain polar residues. TMDs vary greatly in length, sequence, and hydrophobicity, adopting organelle-specific properties. Functions of transmembrane domains Transmembrane domains are known to perform a variety of functions. These include: * Anchoring transmembrane proteins to the membrane. *Facilitating molecular transport of molecules such as ions and proteins across biological membranes; usually hydrophilic residues and binding sites in the TMDs help in this process. * Signal transduction across the membrane; many transmembrane proteins, such as G protein-coupled receptors, receive extracellul ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
G Protein-coupled Receptors
G protein-coupled receptors (GPCRs), also known as seven-(pass)-transmembrane domain receptors, 7TM receptors, heptahelical receptors, serpentine receptors, and G protein-linked receptors (GPLR), form a large group of evolutionarily-related proteins that are cell surface receptors that detect molecules outside the cell and activate cellular responses. Coupling with G proteins, they are called seven-transmembrane receptors because they pass through the cell membrane seven times. Text was copied from this source, which is available under Attribution 2.5 Generic (CC BY 2.5) license. Ligands can bind either to extracellular N-terminus and loops (e.g. glutamate receptors) or to the binding site within transmembrane helices (Rhodopsin-like family). They are all activated by agonists although a spontaneous auto-activation of an empty receptor can also be observed. G protein-coupled receptors are found only in eukaryotes, including yeast, choanoflagellates, and a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhodopsin-like Receptors
Rhodopsin-like receptors are a family of proteins that comprise the largest group of G protein-coupled receptors. Scope G-protein-coupled receptors, GPCRs, constitute a vast protein family that encompasses a wide range of functions (including various autocrine, paracrine, and endocrine processes). They show considerable diversity at the sequence level, on the basis of which they can be separated into distinct groups. GPCRs are usually described as "superfamily" because they embrace a group of families for which there are indications of evolutionary relationship, but between which there is no statistically significant similarity in sequence. The currently known superfamily members include the rhodopsin-like GPCRs (this family), the secretin-like GPCRs, the cAMP receptors, the fungal mating pheromone receptors, and the metabotropic glutamate receptor family. There is a specialised database for GPCRs. Function The rhodopsin-like GPCRs themselves represent a widespread protein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Proteins
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (transmembrane) or associate with one or the other side of a membrane ( integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane. Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs. Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct conformation of the protein in isolation from its native environment. Function Membrane proteins perform a variety of functions vital to the surv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.png)





.png)