|
Olympicene
Olympicene is an organic carbon based molecule formed of five rings, of which four are benzene rings, joined in the shape of the Olympic rings. The molecule was conceived in March 2010 as a way to celebrate the 2012 London Olympics by Graham Richards of University of Oxford and Antony Williams. It was first synthesized by researchers Anish Mistry and David Fox of the University of Warwick in the UK. Relative energies of olympicene and its isomers were first predicted from quantum electronic-structure computations by Andrew Valentine and David Mazziotti of the University of Chicago. Electron counting Olympicene has 18 pi electrons in its ring system; as it is a flat molecule, this makes it an aromatic molecule. The central ring is not an aromatic ring. Related compounds A very similar molecule ( benzo 'c''henanthrene) which lacks the -CH2- spacer between the two sides of the molecule has been known for many years. This earlier molecule has been studied by X-ray crystallograph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Olympiadane
Olympiadane is a mechanically interlocked molecular architectures, mechanically interlocked molecule composed of five interlocking macrocycles that resembles the Olympic symbols, Olympic rings. The molecule is a linear pentacatenane or a [5]catenane. It was synthesized and named by Fraser Stoddart and coworkers in 1994. The molecule was designed without any practical use in mind, although other catenanes may have possible application to the construction of a molecular computer. See also *Olympicene References {{heterocyclic-stub Molecular topology Hexafluorophosphates Supramolecular chemistry Pyridinium compounds Substances discovered in the 1990s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Naphthanthrone
Naphthanthrone is an organic carbon based molecule formed of five rings, of which four are benzene rings, joined in the shape of the Olympic rings. The compound can be synthesized by the condensation of pyrene and glycerol in sulfuric acid. Its crystals belong to the orthorhombic crystal system. See also * Olympicene Olympicene is an organic carbon based molecule formed of five rings, of which four are Benzene#Structure, benzene rings, joined in the shape of the Olympic rings. The molecule was conceived in March 2010 as a way to celebrate the 2012 London Oly ... References Ketones Polycyclic aromatic compounds {{ketone-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bull
A bull is an intact (i.e., not castrated) adult male of the species ''Bos taurus'' (cattle). More muscular and aggressive than the females of the same species (i.e., cows), bulls have long been an important symbol in many religions, including for sacrifices. These animals play a significant role in beef ranching, dairy farming, and a variety of sporting and cultural activities, including bullfighting and bull riding. Due to their temperament, handling requires precautions. Nomenclature The female counterpart to a bull is a cow, while a male of the species that has been castrated is a ''steer'', '' ox'', or ''bullock'', although in North America, this last term refers to a young bull. Use of these terms varies considerably with area and dialect. Colloquially, people unfamiliar with cattle may refer to both castrated and intact animals as "bulls". A wild, young, unmarked bull is known as a ''micky'' in Australia.Sheena Coupe (ed.), ''Frontier Country, Vol. 1'' (Weldon R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group. As such, its systematic IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent. As the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue, toluene is sometimes used as a recreational inhalant and has the potential of causing severe neurological harm. History The compound was first isolated in 1837 through a distillation of pine oil by the Polish chemist Filip Walter, who named it ''rétinnaphte''. In 1841, French chemist Henri Étienne Sainte-Claire Deville isolated a hydrocarbon from balsam of Tolu (an aromatic extract from the tropical Colombian tree ''Myroxylon balsamum''), which Deville recognized as similar to Wa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphonium
In polyatomic cations with the chemical formula (where R is a hydrogen or an alkyl, aryl, or halide group). These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions. Types of phosphonium cations Protonated phosphines The parent phosphonium is as found in the iodide salt, phosphonium iodide. Salts of the parent are rarely encountered, but this ion is an intermediate in the preparation of the industrially useful tetrakis(hydroxymethyl)phosphonium chloride: :PH3 + HCl + 4 CH2O → Many organophosphonium salts are produced by protonation of primary, secondary, and tertiary phosphines: :PR3 + H+ → The basicity of phosphines follows the usual trends, with R = alkyl being more basic than R = aryl. Tetraorganophosphonium cations The most common phosphonium compounds have four organic substituents attached to phosphorus. The quaternary phosphonium cations include tetraphenylphosphonium, (C6H5)4P+ and tetramethylphosphoni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Bromoacetate
Ethyl bromoacetate is the chemical compound with the formula CH2BrCO2C2H5. It is the ethyl ester of bromoacetic acid and is prepared in two steps from acetic acid. It is a lachrymator and has a fruity, pungent odor. It is also a highly toxic alkylating agent and may be fatal if inhaled. Applications Ethyl bromoacetate is listed by the World Health Organization as a riot control agent, and was first employed for that purpose by French police in 1912. The French army used rifle grenades 'grenades lacrymogènes' filled with this gas against the Germans beginning in August 1914, but the weapons were largely ineffective, even though ethyl bromoacetate is twice as toxic as chlorine. In the early months of the war the British also used the weaponized use of tear gas agents and more toxic gasses including sulfur dioxide. The German army then used these attacks to justify their subsequent employment of it as odorant or warning agent in odorless, toxic gases and chemical weapons in 1915 und ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenyl Phosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether. Preparation and structure Triphenylphosphine can be prepared in the laboratory by treatment of phosphorus trichloride with phenylmagnesium bromide or phenyllithium. The industrial synthesis involves the reaction between phosphorus trichloride, chlorobenzene, and sodium: :PCl3 + 3 PhCl + 6 Na → PPh3 + 6 NaCl Triphenylphosphine crystallizes in triclinic and monoclinic modification. In both cases, the molecule adopts a pyramidal structure with propeller-like arrangement of the three phenyl groups. Principal reactions with chalcogens, halogens, and acids Oxidation Triphenylphosphine undergoes sl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
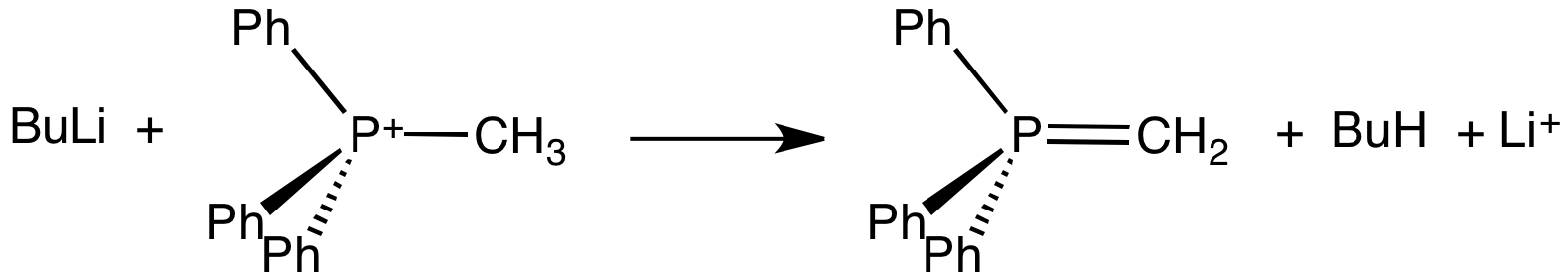
Ylide
An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2-dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates. The class name "ylide" for the compound should not be confused with the suffix "-ylide". Resonance structures Many ylides may be depicted by a multiple bond form in a resonance structure, known as the ylene form, while the actual structure lies in between both forms: : The actual bonding picture of these types of ylides is strictly zwitterionic (the structure on the right) with the strong Coulombic attraction between the " ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrene
Pyrene is a polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings, resulting in a flat aromatic system. The chemical formula is . This yellow solid is the smallest peri-fused PAH (one where the rings are fused through more than one face). Pyrene forms during incomplete combustion of organic compounds. Occurrence and properties Pyrene was first isolated from coal tar, where it occurs up to 2% by weight. As a peri-fused PAH, pyrene is much more resonance-stabilized than its five-member-ring containing isomer fluoranthene. Therefore, it is produced in a wide range of combustion conditions. For example, automobiles produce about 1 μg/km.Senkan, Selim and Castaldi, Marco (2003) "Combustion" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. Reactions Oxidation with chromate affords perinaphthenone and then naphthalene-1,4,5,8-tetracarboxylic acid. Pyrene undergoes a series of hydrogenation reactions and is susceptible to halogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wittig Reaction
The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide called a Wittig reagent. Wittig reactions are most commonly used to convert aldehydes and ketones to alkenes. Most often, the Wittig reaction is used to introduce a methylene group using methylenetriphenylphosphorane (Ph3P=CH2). Using this reagent, even a sterically hindered ketone such as camphor can be converted to its methylene derivative. Stereochemistry For the reaction with aldehydes, the double bond geometry is readily predicted based on the nature of the ylide. With unstabilised ylides (R3 = alkyl) this results in (''Z'')-alkene product with moderate to high selectivity. With stabilized ylides (R3 = ester or ketone), the (''E'')-alkene is formed with high selectivity. The (''E'')/(''Z'') selectivity is often poor with semistabilized ylides (R3 = aryl). To obtain the (''E'')-alkene for unstabilized ylides, the Schlosser modification of the W ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The Periodic Table Of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics in chemistry and related fields. They are published on YouTube and produced by Brady Haran, a former BBC video journalist, mainly featuring Sir Martyn Poliakoff, Peter Licence, Stephen Liddle, Debbie Kays, Neil Barnes, Sam Tang, and other scientists at the University of Nottingham. Development The project began recording on 9 June 2008 and the initial videos were completed on 17 July 2008. The collection includes videos, each just a few minutes long, for all 118 known elements with a video for each element, as well as many additional supplemental chemistry videos. The 118 element videos and introduction videos were all shot unscripted in June and July 2008. Since the initial videos were completed in 2008 the team has been refining and uplo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fraser Stoddart
Sir James Fraser Stoddart (born 24 May 1942) is a British-American chemist who is Board of Trustees Professor of Chemistry and head of the Stoddart Mechanostereochemistry Group in the Department of Chemistry at Northwestern University in the United States. He works in the area of supramolecular chemistry and nanotechnology. Stoddart has developed highly efficient syntheses of mechanically-interlocked molecular architectures such as molecular Borromean rings, catenanes and rotaxanes utilising molecular recognition and molecular self-assembly processes. He has demonstrated that these topologies can be employed as molecular switches. His group has even applied these structures in the fabrication of nanoelectronic devices and nanoelectromechanical systems (NEMS). His efforts have been recognized by numerous awards including the 2007 King Faisal International Prize in Science. He shared the Nobel Prize in Chemistry together with Ben Feringa and Jean-Pierre Sauvage in 2016 for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




.png)
