Phosphonium on:
[Wikipedia]
[Google]
[Amazon]
 In polyatomic cations with the
In polyatomic cations with the
 Quaternary phosphonium cations () are produced by alkylation of organophosphines. For example, the reaction of
Quaternary phosphonium cations () are produced by alkylation of organophosphines. For example, the reaction of
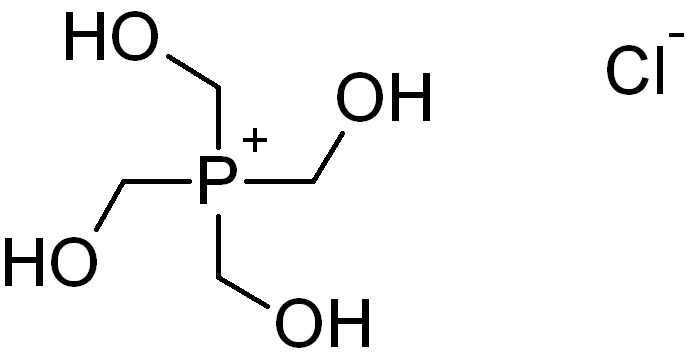 Tetrakis(hydroxymethyl)phosphonium chloride has industrial importance in the production of crease-resistant and
Tetrakis(hydroxymethyl)phosphonium chloride has industrial importance in the production of crease-resistant and
Nitrogen reduction to ammonia at high efficiency and rates based on a phosphonium proton shuttle
/ref>
 In polyatomic cations with the
In polyatomic cations with the chemical formula
In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, ...
(where R is a hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
or an alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl is derived from a cycloal ...
, aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used as ...
, or halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a f ...
group). These cations have tetrahedral structures. The salts
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively ...
are generally colorless or take the color of the anions.
Types of phosphonium cations
Protonated phosphines
The parent phosphonium is as found in the iodide salt, phosphonium iodide. Salts of the parent are rarely encountered, but this ion is an intermediate in the preparation of the industrially useful tetrakis(hydroxymethyl)phosphonium chloride: :PH3 + HCl + 4 CH2O → Many organophosphonium salts are produced by protonation of primary, secondary, and tertiary phosphines: :PR3 + H+ → The basicity of phosphines follows the usual trends, with R = alkyl being more basic than R = aryl.Tetraorganophosphonium cations
The most common phosphonium compounds have four organic substituents attached to phosphorus. The quaternary phosphonium cations include tetraphenylphosphonium, (C6H5)4P+ and tetramethylphosphonium .
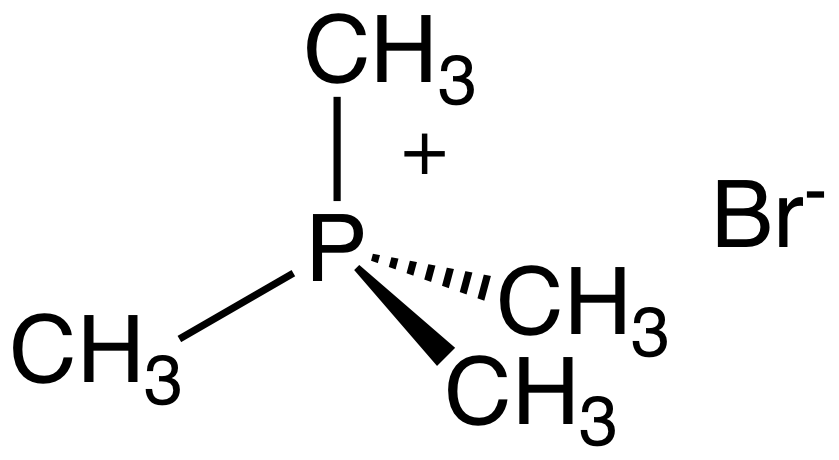
 Quaternary phosphonium cations () are produced by alkylation of organophosphines. For example, the reaction of
Quaternary phosphonium cations () are produced by alkylation of organophosphines. For example, the reaction of triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists ...
with methyl bromide
Bromomethane, commonly known as methyl bromide, is an organobromine compound with formula C H3 Br. This colorless, odorless, nonflammable gas is produced both industrially and biologically. It has a tetrahedral shape and it is a recognized ozo ...
gives methyltriphenylphosphonium bromide:
:PPh3 + CH3Br → H3PPh3sup>+Br−
The methyl group in such phosphonium salts is mildly acidic, with a p''K''a estimated to be near 15:
: H3PPh3sup>+ + base → CH2=PPh3 + basesup>+
This deprotonation reaction gives Wittig reagents.
Phosphorus pentachloride and related compounds
Solidphosphorus pentachloride
Phosphorus pentachloride is the chemical compound with the formula PCl5. It is one of the most important phosphorus chlorides, others being PCl3 and POCl3. PCl5 finds use as a chlorinating reagent. It is a colourless, water-sensitive and moist ...
is an ionic compound
In chemistry, an ionic compound is a chemical compound composed of ions held together by electrostatic forces termed ionic bonding. The compound is neutral overall, but consists of positively charged ions called cations and negatively charged ...
, formulated , that is, a salt containing the tetrachlorophosphonium cation. Dilute solutions dissociate according to the following equilibrium:
:PCl5 + Cl−
Triphenylphosphine dichloride (Ph3PCl2) exists both as the pentacoordinate phosphorane and as the chlorotriphenylphosphonium chloride, depending on the medium. The situation is similar to that of PCl5. It is an ionic compound (PPh3Cl)+Cl− in polar solutions and a molecular species with trigonal bipyramidal molecular geometry in apolar solution.
Alkoxyphosphonium salts: Arbuzov reaction
The Michaelis–Arbuzov reaction is thechemical reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and break ...
of a trivalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of a ...
phosphorus ester with an alkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely us ...
to form a pentavalent phosphorus species and another alkyl halide. Commonly, the phosphorus substrate is a phosphite ester (P(OR)3) and the alkylating agent is an alkyl iodide.
center, 600px, The mechanism of the Michaelis–Arbuzov reaction
Uses
Textile finishes
 Tetrakis(hydroxymethyl)phosphonium chloride has industrial importance in the production of crease-resistant and
Tetrakis(hydroxymethyl)phosphonium chloride has industrial importance in the production of crease-resistant and flame-retardant
The term flame retardants subsumes a diverse group of chemicals that are added to manufactured materials, such as plastics and textiles, and surface finishes and coatings. Flame retardants are activated by the presence of an ignition source and ...
finishes on cotton textiles and other cellulosic fabrics.Svara, Jürgen; Weferling, Norbert ; Hofmann, Thomas. Phosphorus Compounds, Organic. Ullmann's Encyclopedia of Industrial Chemistry. John Wiley & Sons, Inc., 2008 A flame-retardant finish can be prepared from THPC by the Proban Process, in which THPC is treated with urea. The urea
Urea, also known as carbamide, is an organic compound with chemical formula . This amide has two amino groups (–) joined by a carbonyl functional group (–C(=O)–). It is thus the simplest amide of carbamic acid.
Urea serves an important ...
condenses with the hydroxymethyl groups on THPC. The phosphonium structure is converted to phosphine oxide
Phosphine oxides are phosphorus compounds with the formula OPX3. When X = alkyl or aryl, these are organophosphine oxides. Triphenylphosphine oxide is an example. An inorganic phosphine oxide is phosphoryl chloride (POCl3).
Structure and bonding ...
as the result of this reaction.
Phase-transfer catalysts and precipitating agents
Organic phosphonium cations are lipophilic and can be useful in phase transfer catalysis, much like quaternary ammonium salts. Salts or inorganic anions and tetraphenylphosphonium () are soluble in polar organic solvents. One example is the perrhenate (PPh4 eO4.Reagents for organic synthesis
Wittig reagents are used inorganic synthesis
Organic synthesis is a special branch of chemical synthesis and is concerned with the intentional construction of organic compounds. Organic molecules are often more complex than inorganic compounds, and their synthesis has developed into one o ...
. They are derived from phosphonium salts. A strong base such as butyllithium or sodium amide is required for the deprotonation:
: h3P+CH2R− + C4H9Li → Ph3P=CHR + LiX + C4H10
One of the simplest ylides is methylenetriphenylphosphorane (Ph3P=CH2).. Describes Ph3P=CH2.
The compounds Ph3PX2 (X = Cl, Br) are used in the Kirsanov reaction The Kirsanov reaction is a method for the synthesis of certain organophosphorus compounds. In this reaction a tertiary phosphine is combined with a halogen and then an amine to give the iminophosphines, which are useful ligands and useful reagents ...
.
The Kinnear–Perren reaction is used to prepare alkylphosphonyl dichlorides (RP(O)Cl2) and esters (RP(O)(OR′)2). A key intermediate are alkyltrichlorophosphonium salts, obtained by the alkylation of phosphorus trichloride
Phosphorus trichloride is an inorganic compound with the chemical formula PCl3. A colorless liquid when pure, it is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic a ...
:
:RCl + PCl3 + AlCl3 → PCl3sup>+
Ammonia production for "green hydrogen"
The main industrial procedure for the production of ammonia today is the thermal Haber-Bosch process, which generally uses fossil gas as a source of hydrogen, which is then combined with nitrogen to produce ammonia. In 2021, Professor Doug MacFarlane and collaborators Alexandr Simonov and Bryan Suryanto ofMonash University
Monash University () is a public research university based in Melbourne, Victoria, Australia. Named for prominent World War I general Sir John Monash, it was founded in 1958 and is the second oldest university in the state. The university h ...
devised a method of producing green ammonia that has the potential to make Haber-Bosch plants obsolete. Their process is similar to the electrolysis approach for producing hydrogen. While working with local company Verdant, which wanted to make bleach from saltwater by electrolysis, Suryanto discovered that a tetraalkyl phosphonium salt allowed the efficient production of ammonia at room temperature./ref>
See also
*Ammonium
The ammonium cation is a positively-charged polyatomic ion with the chemical formula or . It is formed by the protonation of ammonia (). Ammonium is also a general name for positively charged or protonated substituted amines and quaternar ...
()
*Hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the common name for the aqueous cation , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is di ...
(H3O+)
* Onium compounds
* Organophosphorus chemistry
References
{{Reflist Cations Functional groups Phosphonium compounds