|
Methylenetriphenylphosphorane
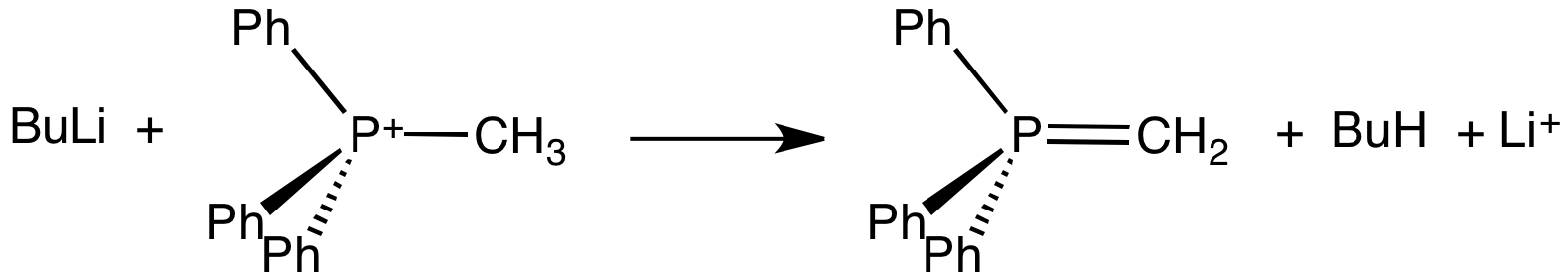
Methylenetriphenylphosphorane is an organophosphorus compound with the formula Ph3PCH2. It is the parent member of the phosphorus ylides, popularly known as Wittig reagents. It is a highly polar, highly basic species. Preparation and use Methylenetriphenylphosphorane is prepared from methyltriphenylphosphonium bromide by its deprotonation using a strong base like butyllithium: :Ph3PCH3Br + BuLi → Ph3PCH2 + LiBr + BuH The phosphorane is generally not isolated, instead it is used in situ. The estimated pKa of this carbon acid is near 15. Potassium tert-butoxide has been used in place of butyl lithium. Sodium amide has also been used a base. Methylenetriphenylphosphorane is used to replace oxygen centres in aldehydes and ketones with a methylene group, i.e., a methylenation: :R2CO + Ph3PCH2 → R2C=CH2 + Ph3PO The phosphorus-containing product is triphenylphosphine oxide. Structure Crystallographic characterization of the colourless ylide reveals that the phosphorus atom is approx ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbomethoxymethylenetriphenylphosphorane
Carbomethoxymethylenetriphenylphosphorane is a chemical compound used in organic syntheses. It contains a phosphorus atom bound to three phenyl groups, and doubly bound to the alpha position of methyl acetate. It undergoes a Wittig reaction. It is used in the Vitamin B12 total synthesis. Production Carbomethoxymethylenetriphenylphosphorane can be made via a multistep reaction using bromoacetic acid, dicyclohexylcarbodiimide, and triphenylphosphine. This makes a phosphonium salt, which is converted to the final product by sodium carbonate in water. Reactions Carbomethoxymethylenetriphenylphosphorane reacts with aldehyde In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...s to give a two carbon atom extension. The carbomethoxymethylene group replaces the oxygen of the aldehyde to give ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wittig Reagent
In organic chemistry, Wittig reagents are organophosphorus compounds of the formula R3P=CHR', where R is usually phenyl. They are used to convert ketones and aldehydes to alkenes: : Preparation Because they typically hydrolyze and oxidize readily, Wittig reagents are prepared using air-free techniques. They are typically generated and used in situ. THF is a typical solvent. Some are sufficiently stable to be sold commercially. ;Formation of phosphonium salt Wittig reagents are usually prepared from a phosphonium salt, which is in turn prepared by the quaternization of triphenylphosphine with an alkyl halide. Wittig reagents are usually derived from a primary alkyl halide. Quaternization of triphenylphosphine with secondary halides is typically inefficient. For this reason, Wittig reagents are rarely used to prepare tetrasubstituted alkenes. ;Bases for deprotonation of phosphonium salts The alkylphosphonium salt is deprotonated with a strong base such as ''n''-butyllithium: : ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylenation
In organic chemistry, methylenation is a chemical reaction that inserts a methylene () group into a chemical compound: :\ce + \longrightarrow \ce\ce Typically, the reaction is used to prepare terminal alkenes from aldehydes and, less frequently, ketones. Methods Methylene-for-oxo reactions A common method for methylenation involves the Wittig reaction using methylenetriphenylphosphorane with an aldehyde (Ph = phenyl, ): :RCHO + Ph3P=CH2 -> RCH=CH2 + Ph3PO A related reaction can be accomplished with Tebbe's reagent, which is sufficiently versatile to allow methylenation of esters: :RCO2R' + Cp2Ti(Cl)CH2AlMe2 -> RC(OR')=CH2 + Cp2TiOAlMe2Cl Other less well-defined titanium reagents, e.g., Lombardo's reagent, effect similar transformations. : Carbanions derived from methylsulfones have also been employed, equivalently to the Wittig reaction. Other approaches Ethenolysis is a method for methylenation of internal alkene as illustrated by the following example: :\ce + \longrighta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyltriphenylphosphonium Bromide
Methyltriphenylphosphonium bromide is the organophosphorus compound with the formula C6H5)3PCH3r. It is the bromide salt of a phosphonium cation. It is a white salt that is soluble in polar organic solvents. Synthesis and reactions Methyltriphenylphosphonium bromide is produced by treating triphenylphosphine with methyl bromide: :Ph3P + CH3Br → Ph3PCH3Br Methyltriphenylphosphonium bromide is the principal precursor to methylenetriphenylphosphorane Methylenetriphenylphosphorane is an organophosphorus compound with the formula Ph3PCH2. It is the parent member of the phosphorus ylides, popularly known as Wittig reagents. It is a highly polar, highly basic species. Preparation and use Methylen ..., a useful methylenating reagent. This conversion is achieved by treating methyltriphenylphosphonium bromide with strong base.{{cite journal, doi = 10.1080/00397918508063883, title = The Wittig Reaction Using Potassium-tert-butoxide High Yield Methylenations of Sterically Hindered K ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
(Chloromethylene)triphenylphosphorane
(Chloromethylene)triphenylphosphorane is the organophosphorus compound with he formula Ph3P=CHCl (Ph = phenyl). It is a white solid but is usually generated and used in situ as a reagent in organic synthesis. It is structurally and chemically related to methylenetriphenylphosphorane. The reagent is prepared from the chloromethylphosphonium salt h3PCH2Cll by treatment with strong base. The phosphonium compound is generated by treatment of triphenylphosphine with chloroiodomethane. (Chloromethylene)triphenylphosphorane converts aldehydes to vinyl chlorides: :RCHO + Ph3P=CHCl → RCH=CHCl + Ph3PO These vinyl chlorides undergo dehydrochlorination to give alkyne \ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and n ...s: :RCH=CHCl + NaN(SiMe3)2 → RC≡CH + NaCl + HN(SiMe3)2 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ylide
An ylide or ylid () is a neutral dipolar molecule containing a formally negatively charged atom (usually a carbanion) directly attached to a heteroatom with a formal positive charge (usually nitrogen, phosphorus or sulfur), and in which both atoms have full octets of electrons. The result can be viewed as a structure in which two adjacent atoms are connected by both a covalent and an ionic bond; normally written X+–Y−. Ylides are thus 1,2-dipolar compounds, and a subclass of zwitterions. They appear in organic chemistry as reagents or reactive intermediates. The class name "ylide" for the compound should not be confused with the suffix "-ylide". Resonance structures Many ylides may be depicted by a multiple bond form in a resonance structure, known as the ylene form, while the actual structure lies in between both forms: : The actual bonding picture of these types of ylides is strictly zwitterionic (the structure on the right) with the strong Coulombic attraction between the " ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methoxymethylenetriphenylphosphine
Methoxymethylenetriphenylphosphine is a Wittig reagent used for the homologization of aldehydes, and ketones to extended aldehydes, a organic reaction first reported in 1958. The reagent is generally prepared and used in situ. It has blood-red color, indicative of destabilized ylides. Preparation The reagent can be prepared in two steps from triphenylphosphine. The first step is ''P''-alkylation with chloromethyl methyl ether. : In the second step, the resulting phosphonium salt is deprotonated. : In place of chloromethyl methyl ether, a mixture of methylal and acetyl chloride can be used. Uses This reagent reacts with a ketone or aldehyde in a Wittig reaction to give an enol ether, which can be converted to the aldehyde by acid-induced hydrolysis. The initial report of the reaction demonstrated its use on the steroid tigogenone. : It was later used in the Wender Taxol total synthesis and the Stork quinine total synthesis. References # {{Note, Levine ''A new aldehyde synthe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chem
Chem may refer to: * Chemistry practical waali mam *Chemistry *Chemical * ''Chem'' (journal), a scientific journal published by Cell Press *Post apocalyptic slang for "drugs", medicinal or otherwise in the Fallout video game series. In Ancient Egyptian usage: * ''Khem'' (also spelt ''Chem''), the Egyptian word for "black" * Min (god), in the past erroneously named ''Khem'' CHEM may refer to : *A metabolic panel: for instance, CHEM-7, which is the basic metabolic panel *CHEM-DT CHEM-DT is the TVA owned-and-operated television station in Trois-Rivières, Quebec, Canada. It broadcasts a high-definition digital signal on VHF channel 8 from a transmitter on Rue Principale in Notre-Dame-du-Mont-Carmel. Owned by the Grou ..., a Canadian television channel See also * Chemo (other) * Kemi, a place in Finland {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wittig CH2 Examples
Wittig is a surname, and may refer to: * Burghardt Wittig (born 1947), German biochemist * Curt Wittig, American chemist * David Wittig (born 1955), American executive * Edward Wittig (1879–1941), Polish sculptor * Ferdinand Wittig (1851-1909), American politician * Georg Wittig (1897–1987), German chemist * Iris Wittig (1928-1978), German military pilot * Johnnie Wittig (1914–1999), former Major League pitcher * Martin C. Wittig (born 1964), German CEO * Michael Wittig (born 1976), American musician * Monique Wittig (1935–2003), French author and feminist theorist * Peter Wittig (born 1954), German diplomat * Rüdiger Wittig (born 1946), German professor of geobotany and ecology * Sigmar Wittig (born 1940), German Chair of the European Space Agency * Susan Wittig Albert (born 1940), American mystery writer See also * Wittich * Wittig River * 1,2-Wittig rearrangement * 2,3-Wittig rearrangement * Wittig reaction The Wittig reaction or Wittig olefination is a chemica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resonance Structure
In chemistry, resonance, also called mesomerism, is a way of describing Chemical bond, bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ''canonical structures'') into a resonance hybrid (or ''hybrid structure'') in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. Overview Under the framework of valence bond theory, resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. For many chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is sufficient for describing the chemical bonding and rationalizing experimentally determined molecular properties like Bond length, bond lengths, Bond angl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylphosphine Oxide
Triphenylphosphine oxide (often abbreviated TPPO) is the organophosphorus compound with the formula OP(C6H5)3, also written as Ph3PO or PPh3O (Ph = phenyl, C6H5). This colourless crystalline compound is a common but potentially useful waste product in reactions involving triphenylphosphine. It is a popular reagent to induce the crystallization, crystallizing of chemical compounds. Structure and properties Ph3PO is a tetrahedral molecule related to POCl3. The oxygen center is relatively basic. The rigidity of the backbone and the basicity of the oxygen center make this species a popular agent to crystallize otherwise difficult to crystallize molecules. This trick is applicable to molecules that have acidic hydrogen atoms, e.g. phenols. Up to now, several modifications of Ph3PO have been found: For example, a monoclinic form crystalizes in the space group ''P''21/''c'' with Z = 4 and a = 15.066(1) Å, b = 9.037(2) Å, c = 11.296(3) Å, and β = 98.47(1)°. The orthorhombic modif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organophosphorus Compound
Organophosphorus compounds are organic compounds containing phosphorus. They are used primarily in pest control as an alternative to chlorinated hydrocarbons that persist in the environment. Some organophosphorus compounds are highly effective insecticides, although some are extremely toxic to humans, including sarin and VX nerve agents. Organophosphorus chemistry is the corresponding science of the properties and reactivity of organophosphorus compounds. Phosphorus, like nitrogen, is in group 15 of the periodic table, and thus phosphorus compounds and nitrogen compounds have many similar properties. The definition of organophosphorus compounds is variable, which can lead to confusion. In industrial and environmental chemistry, an organophosphorus compound need contain only an organic substituent, but need not have a direct phosphorus-carbon (P-C) bond. Thus a large proportion of pesticides (e.g., malathion), are often included in this class of compounds. Phosphorus can adopt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



nickel(II)-from-xtal-3D-balls.png)