|
OBSCN
Obscurin is a protein that in humans is encoded by the ''OBSCN'' gene. Obscurin belongs to the family of giant sarcomeric signaling proteins that includes titin and nebulin. Obscurin is expressed in cardiac and skeletal muscle, and plays a role in the organization of myofibrils during sarcomere assembly. A mutation in the ''OBSCN'' gene has been associated with hypertrophic cardiomyopathy and altered obscurin protein properties have been associated with other muscle diseases. Structure Human obscurin may exist as multiple splice variants of approximately 720 kDa, however the full-length nature of only one has been described to date. Obscurin is expressed in cardiac and skeletal muscle. The obscurin gene spans more than 150 kb, contains over 80 exons. The encoded protein contains 68 Ig domains, 2 fibronectin domains, 1 calcium/calmodulin-binding domain, 1 RhoGEF domain with an associated PH domain, and 2 serine-threonine kinase domains. The dominant location of obscurin in mature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ANK2
Ankyrin-2, also known as Ankyrin-B, and Brain ankyrin, is a protein which in humans is encoded by the ''ANK2'' gene. Ankyrin-2 is ubiquitously expressed, but shows high expression in cardiac muscle. Ankyrin-2 plays an essential role in the localization and membrane stabilization of ion transporters and ion channels in cardiomyocytes, as well as in costamere structures. Mutations in ''ANK2'' cause a dominantly-inherited, cardiac arrhythmia syndrome known as long QT syndrome 4 as well as sick sinus syndrome; mutations have also been associated to a lesser degree with hypertrophic cardiomyopathy. Alterations in ankyrin-2 expression levels are observed in human heart failure. Structure Ankyrin-B protein is around 220 kDa, with several isoforms. The ''ANK2'' gene is approximately 560 kb in size and consists of 53 exons on human chromosome 4; ''ANK2'' is also transcriptionally regulated via over 30 alternative splicing events with variable expression of isoforms in cardiac muscle. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ANK1
Ankyrin 1, also known as ANK-1, and erythrocyte ankyrin, is a protein that in humans is encoded by the ''ANK1'' gene. Tissue distribution The protein encoded by this gene, Ankyrin 1, is the prototype of the ankyrin family, was first discovered in erythrocytes, but since has also been found in brain and muscles. Genetics Complex patterns of alternative splicing in the regulatory domain, giving rise to different isoforms of ankyrin 1 have been described, however, the precise functions of the various isoforms are not known. Alternative polyadenylation accounting for the different sized erythrocytic ankyrin 1 mRNAs, has also been reported. Truncated muscle-specific isoforms of ankyrin 1 resulting from usage of an alternate promoter have also been identified. Disease linkage Mutations in erythrocytic ankyrin 1 have been associated in approximately half of all patients with hereditary spherocytosis. ANK1 shows altered methylation and expression in Alzheimer's disease. A gene expre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
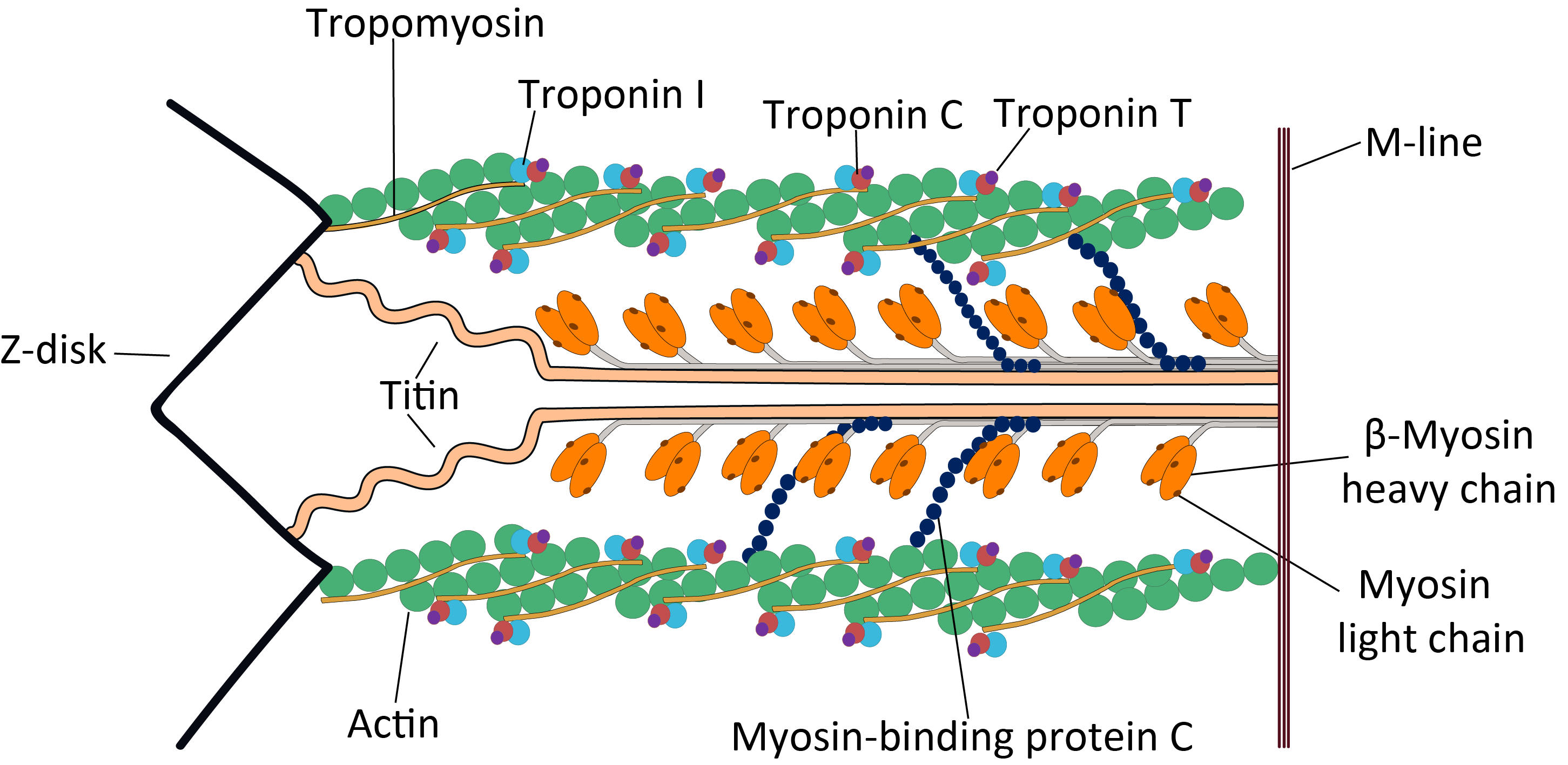
Titin
Titin (contraction for Titan protein) (also called connectin) is a protein that in humans is encoded by the ''TTN'' gene. Titin is a giant protein, greater than 1 µm in length, that functions as a molecular spring that is responsible for the passive elasticity of muscle. It comprises 244 individually folded protein domains connected by unstructured peptide sequences. These domains unfold when the protein is stretched and refold when the tension is removed. Titin is important in the contraction of striated muscle tissues. It connects the Z line to the M line in the sarcomere. The protein contributes to force transmission at the Z line and resting tension in the I band region. It limits the range of motion of the sarcomere in tension, thus contributing to the passive stiffness of muscle. Variations in the sequence of titin between different types of striated muscle (cardiac or skeletal) have been correlated with differences in the mechanical properties of these muscles. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titin
Titin (contraction for Titan protein) (also called connectin) is a protein that in humans is encoded by the ''TTN'' gene. Titin is a giant protein, greater than 1 µm in length, that functions as a molecular spring that is responsible for the passive elasticity of muscle. It comprises 244 individually folded protein domains connected by unstructured peptide sequences. These domains unfold when the protein is stretched and refold when the tension is removed. Titin is important in the contraction of striated muscle tissues. It connects the Z line to the M line in the sarcomere. The protein contributes to force transmission at the Z line and resting tension in the I band region. It limits the range of motion of the sarcomere in tension, thus contributing to the passive stiffness of muscle. Variations in the sequence of titin between different types of striated muscle (cardiac or skeletal) have been correlated with differences in the mechanical properties of these muscles. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunoglobulin Domain
The immunoglobulin domain, also known as the immunoglobulin fold, is a type of protein domain that consists of a 2-layer sandwich of 7-9 antiparallel β-strands arranged in two β-sheets with a Greek key topology, consisting of about 125 amino acids. The backbone switches repeatedly between the two β-sheets. Typically, the pattern is (N-terminal β-hairpin in sheet 1)-(β-hairpin in sheet 2)-(β-strand in sheet 1)-(C-terminal β-hairpin in sheet 2). The cross-overs between sheets form an "X", so that the N- and C-terminal hairpins are facing each other. Members of the immunoglobulin superfamily are found in hundreds of proteins of different functions. Examples include antibodies, the giant muscle kinase titin, and receptor tyrosine kinases. Immunoglobulin-like domains may be involved in protein–protein and protein–ligand interactions. Examples Human genes encoding proteins containing the immunoglobulin domain include: * A1BG * ACAM * ADAMTSL1 * ADAMTSL3 * AGER * A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RhoGEF Domain
RhoGEF domain describes two distinct structural domains with guanine nucleotide exchange factor (GEF) activity to regulate small GTPases in the Rho family. Rho small GTPases are inactive when bound to GDP but active when bound to GTP; RhoGEF domains in proteins are able to promote GDP release and GTP binding to activate specific Rho family members, including RhoA, Rac1 and Cdc42. The largest class of RhoGEFs is composed of proteins containing the " Dbl-homology" (DH) domain, which almost always is found together with a pleckstrin-homology (PH) domain to form a combined DH/PH domain structure. A distinct class of RhoGEFs is those proteins containing the DOCK/CZH/DHR-2 domain. This structure has no sequence similarity with DBL-homology domains. Human proteins containing DH/PH RhoGEF domain ABR; AKAP13/ARHGEF13/Lbc; ALS2; ALS2CL; ARHGEF1/p115-RhoGEF; ARHGEF10; ARHGEF10L; ARHGEF11/PDZ-RhoGEF.; ARHGEF12/LARG; ARHGEF15; ARHGEF16; ARHGEF17; ARHGEF18; ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pleckstrin Homology Domain
Pleckstrin homology domain (PH domain) or (PHIP) is a protein domain of approximately 120 amino acids that occurs in a wide range of proteins involved in intracellular signaling or as constituents of the cytoskeleton. This domain can bind phosphatidylinositol lipids within biological membranes (such as phosphatidylinositol (3,4,5)-trisphosphate and phosphatidylinositol (4,5)-bisphosphate), and proteins such as the βγ-subunits of heterotrimeric G proteins, and protein kinase C. Through these interactions, PH domains play a role in recruiting proteins to different membranes, thus targeting them to appropriate cellular compartments or enabling them to interact with other components of the signal transduction pathways. Lipid binding specificity Individual PH domains possess specificities for phosphoinositides phosphorylated at different sites within the inositol ring, e.g., some bind phosphatidylinositol (4,5)-bisphosphate but not phosphatidylinositol (3,4,5)-trisphosphate or p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule (functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis bases. The nature of metal–ligand bonding can range from covalent to ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cardiac
The heart is a muscular organ in most animals. This organ pumps blood through the blood vessels of the circulatory system. The pumped blood carries oxygen and nutrients to the body, while carrying metabolic waste such as carbon dioxide to the lungs. In humans, the heart is approximately the size of a closed fist and is located between the lungs, in the middle compartment of the chest. In humans, other mammals, and birds, the heart is divided into four chambers: upper left and right atria and lower left and right ventricles. Commonly the right atrium and ventricle are referred together as the right heart and their left counterparts as the left heart. Fish, in contrast, have two chambers, an atrium and a ventricle, while most reptiles have three chambers. In a healthy heart blood flows one way through the heart due to heart valves, which prevent backflow. The heart is enclosed in a protective sac, the pericardium, which also contains a small amount of fluid. The wall o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exon
An exon is any part of a gene that will form a part of the final mature RNA produced by that gene after introns have been removed by RNA splicing. The term ''exon'' refers to both the DNA sequence within a gene and to the corresponding sequence in RNA transcripts. In RNA splicing, introns are removed and exons are covalently joined to one another as part of generating the mature RNA. Just as the entire set of genes for a species constitutes the genome, the entire set of exons constitutes the exome. History The term ''exon'' derives from the expressed region and was coined by American biochemist Walter Gilbert in 1978: "The notion of the cistron… must be replaced by that of a transcription unit containing regions which will be lost from the mature messengerwhich I suggest we call introns (for intragenic regions)alternating with regions which will be expressedexons." This definition was originally made for protein-coding transcripts that are spliced before being translated. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |

4-3D-balls.png)
