|
ANK1
Ankyrin 1, also known as ANK-1, and erythrocyte ankyrin, is a protein that in humans is encoded by the ''ANK1'' gene. Tissue distribution The protein encoded by this gene, Ankyrin 1, is the prototype of the ankyrin family, was first discovered in erythrocytes, but since has also been found in brain and muscles. Genetics Complex patterns of alternative splicing in the regulatory domain, giving rise to different isoforms of ankyrin 1 have been described, however, the precise functions of the various isoforms are not known. Alternative polyadenylation accounting for the different sized erythrocytic ankyrin 1 mRNAs, has also been reported. Truncated muscle-specific isoforms of ankyrin 1 resulting from usage of an alternate promoter have also been identified. Disease linkage Mutations in erythrocytic ankyrin 1 have been associated in approximately half of all patients with hereditary spherocytosis. ANK1 shows altered methylation and expression in Alzheimer's disease. A gene expre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ankyrin
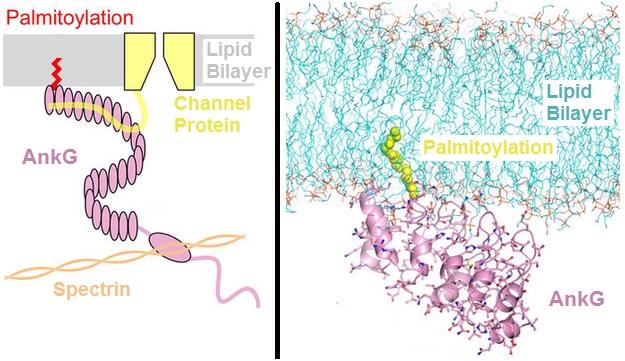
Ankyrins are a family of proteins that mediate the attachment of integral membrane proteins to the spectrin-actin based membrane cytoskeleton. Ankyrins have binding sites for the beta subunit of spectrin and at least 12 families of integral membrane proteins. This linkage is required to maintain the integrity of the plasma membranes and to anchor specific ion channels, ion exchangers and ion transporters in the plasma membrane. The name is derived from the Greek word ἄγκυρα (''ankyra'') for "anchor". Structure Ankyrins contain four functional domains: an N-terminal domain that contains 24 tandem ankyrin repeats, a central domain that binds to spectrin, a death domain that binds to proteins involved in apoptosis, and a C-terminal regulatory domain that is highly variable between different ankyrin proteins. Membrane protein recognition The 24 tandem ankyrin repeats are responsible for the recognition of a wide range of membrane proteins. These 24 repeats contain 3 str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ankyrin
Ankyrins are a family of proteins that mediate the attachment of integral membrane proteins to the spectrin-actin based membrane cytoskeleton. Ankyrins have binding sites for the beta subunit of spectrin and at least 12 families of integral membrane proteins. This linkage is required to maintain the integrity of the plasma membranes and to anchor specific ion channels, ion exchangers and ion transporters in the plasma membrane. The name is derived from the Greek word ἄγκυρα (''ankyra'') for "anchor". Structure Ankyrins contain four functional domains: an N-terminal domain that contains 24 tandem ankyrin repeats, a central domain that binds to spectrin, a death domain that binds to proteins involved in apoptosis, and a C-terminal regulatory domain that is highly variable between different ankyrin proteins. Membrane protein recognition The 24 tandem ankyrin repeats are responsible for the recognition of a wide range of membrane proteins. These 24 repeats contain 3 str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
OBSCN
Obscurin is a protein that in humans is encoded by the ''OBSCN'' gene. Obscurin belongs to the family of giant sarcomeric signaling proteins that includes titin and nebulin. Obscurin is expressed in cardiac and skeletal muscle, and plays a role in the organization of myofibrils during sarcomere assembly. A mutation in the ''OBSCN'' gene has been associated with hypertrophic cardiomyopathy and altered obscurin protein properties have been associated with other muscle diseases. Structure Human obscurin may exist as multiple splice variants of approximately 720 kDa, however the full-length nature of only one has been described to date. Obscurin is expressed in cardiac and skeletal muscle. The obscurin gene spans more than 150 kb, contains over 80 exons. The encoded protein contains 68 Ig domains, 2 fibronectin domains, 1 calcium/calmodulin-binding domain, 1 RhoGEF domain with an associated PH domain, and 2 serine-threonine kinase domains. The dominant location of obscurin in mature ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RHAG
Rh-associated glycoprotein (RHAG) is an ammonia transporter protein that in humans is encoded by the ''RHAG'' gene. RHAG has also recently been designated CD241 (cluster of differentiation 241). Mutations in the RHAG gene can cause stomatocytosis. Function The Rh blood group antigens (MIM 111700) are associated with human erythrocyte membrane proteins of approximately 30 kD, the so-called Rh30 polypeptides. Heterogeneously glycosylated membrane proteins of 50 and 45 kD, the Rh50 glycoproteins, are coprecipitated with the Rh30 polypeptides on immunoprecipitation with anti-Rh-specific mono- and polyclonal antibodies. The Rh antigens appear to exist as a multisubunit complex of CD47 (MIM 601028), LW (MIM 111250), glycophorin B (MIM 111740), and play a critical role in the Rh50 glycoprotein upplied by OMIM Interactions RHAG has been shown to interact with ANK1. See also * Rh deficiency syndrome References Further reading * * * * * * * * * * * * * * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titin
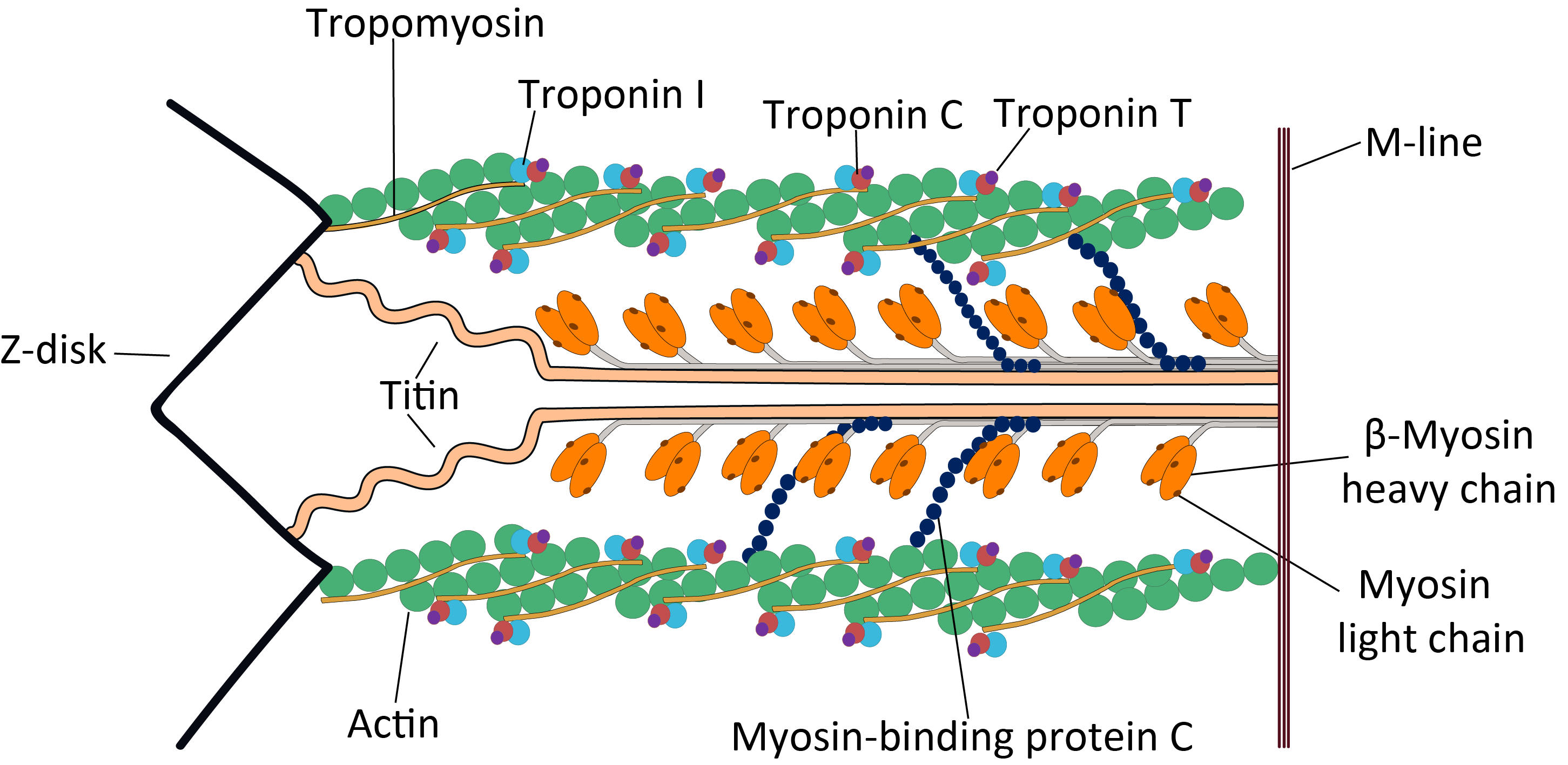
Titin (contraction for Titan protein) (also called connectin) is a protein that in humans is encoded by the ''TTN'' gene. Titin is a giant protein, greater than 1 µm in length, that functions as a molecular spring that is responsible for the passive elasticity of muscle. It comprises 244 individually folded protein domains connected by unstructured peptide sequences. These domains unfold when the protein is stretched and refold when the tension is removed. Titin is important in the contraction of striated muscle tissues. It connects the Z line to the M line in the sarcomere. The protein contributes to force transmission at the Z line and resting tension in the I band region. It limits the range of motion of the sarcomere in tension, thus contributing to the passive stiffness of muscle. Variations in the sequence of titin between different types of striated muscle ( cardiac or skeletal) have been correlated with differences in the mechanical properties of these muscle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
T-cell Lymphoma Invasion And Metastasis-inducing Protein 1
T-cell lymphoma invasion and metastasis-inducing protein 1 is a protein that in humans is encoded by the ''TIAM1'' gene. Structure TIAM1 is tightly associate with BAIAP2 as a subunit. It contains one DH (DBL-homology) domain, one PDZ domain, two PH domains and one Ras-binding RBD domain. Function TIAM1 modulates the activity of Rho GTP-binding proteins and connects extracellular signals to cytoskeletal activities. In addition, TIAM1 activates Rac1, CDC42, and to a lesser extent RhoA. Clinical significance TIAM1 is found in virtually all tumor cell lines examined including B- and T-lymphomas, neuroblastomas, melanomas and carcinomas. Interactions T-cell lymphoma invasion and metastasis-inducing protein 1 has been shown to interact with ANK1, Myc, RAC1 and PPP1R9B. Tiam1 interacts also with para-cingulin Cingulin (CGN; from the Latin ''cingere'' “to form a belt around”) is a cytosolic protein encoded by the ''CGN'' gene in humans localized at tight junct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoskeleton
The cytoskeleton is a complex, dynamic network of interlinking protein filaments present in the cytoplasm of all cells, including those of bacteria and archaea. In eukaryotes, it extends from the cell nucleus to the cell membrane and is composed of similar proteins in the various organisms. It is composed of three main components, microfilaments, intermediate filaments and microtubules, and these are all capable of rapid growth or disassembly dependent on the cell's requirements. A multitude of functions can be performed by the cytoskeleton. Its primary function is to give the cell its shape and mechanical resistance to deformation, and through association with extracellular connective tissue and other cells it stabilizes entire tissues. The cytoskeleton can also contract, thereby deforming the cell and the cell's environment and allowing cells to migrate. Moreover, it is involved in many cell signaling pathways and in the uptake of extracellular material ( endocytosis), ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Striated Muscle
Striations means a series of ridges, furrows or linear marks, and is used in several ways: * Glacial striation * Striation (fatigue), in material * Striation (geology), a ''striation'' as a result of a geological fault * Striation Valley, in Antarctica * In hyperbolic geometry, a ''striation'' is a reflection across two parallel mirrors. * In anatomy, striated muscle * Striations can be found in certain glasses. These have been caused by turbulent flow during teeming (pouring) of the glass. * Striations can be observed in clouds In meteorology, a cloud is an aerosol consisting of a visible mass of miniature liquid drop (liquid), droplets, ice crystals, frozen crystals, or other particulates, particles suspended in the atmosphere of a planetary body or similar space. .... See Barber's pole. * Ballistic fingerprinting {{disambiguation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxy Terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often conta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that j ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |