|
Narrow-gap Semiconductor
Narrow-gap semiconductors are semiconducting materials with a band gap that is comparatively small compared to that of silicon, i.e. smaller than 1.11 eV at room temperature. They are used as infrared detectors or thermoelectrics. List of narrow-gap semiconductors : See also * List of semiconductor materials Semiconductor materials are nominally small band gap insulators. The defining property of a semiconductor material is that it can be compromised by doping it with impurities that alter its electronic properties in a controllable way. Because of ... * Wide-bandgap semiconductor References * Dornhaus, R., Nimtz, G., Schlicht, B. (1983). ''Narrow-Gap Semiconductors''. Springer Tracts in Modern Physics 98, (print) (online) * Semiconductor material types {{CMP-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Semiconducting
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way. Its conducting properties may be altered in useful ways by introducing impurities (" doping") into the crystal structure. When two differently doped regions exist in the same crystal, a semiconductor junction is created. The behavior of charge carriers, which include electrons, ions, and electron holes, at these junctions is the basis of diodes, transistors, and most modern electronics. Some examples of semiconductors are silicon, germanium, gallium arsenide, and elements near the so-called "metalloid staircase" on the periodic table. After silicon, gallium arsenide is the second-most common semiconductor and is used in laser diodes, solar cells, microwave-frequency integrated circuits, and others. Silicon is a critical element for f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium Arsenide
Indium arsenide, InAs, or indium monoarsenide, is a narrow-bandgap semiconductor composed of indium and arsenic. It has the appearance of grey cubic crystals with a melting point of 942 °C. Indium arsenide is similar in properties to gallium arsenide and is a direct bandgap material, with a bandgap of 0.35 eV at room temperature. Indium arsenide is used for construction of infrared detectors, for the wavelength range of 1–3.8 µm. The detectors are usually photovoltaic photodiodes. Cryogenically cooled detectors have lower noise, but InAs detectors can be used in higher-power applications at room temperature as well. Indium arsenide is also used for making of diode lasers. InAs is well known for its high electron mobility and narrow energy bandgap. It is widely used as terahertz radiation source as it is a strong photo-Dember emitter. The optoelectronic properties and phonon vibrations are slightly changed under the effect of temperature over the range form 0 K ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Semiconductor Materials
Semiconductor materials are nominally small band gap insulators. The defining property of a semiconductor material is that it can be compromised by doping it with impurities that alter its electronic properties in a controllable way. Because of their application in the computer and photovoltaic industry—in devices such as transistors, lasers, and solar cells—the search for new semiconductor materials and the improvement of existing materials is an important field of study in materials science. Most commonly used semiconductor materials are crystalline inorganic solids. These materials are classified according to the periodic table groups of their constituent atoms. Different semiconductor materials differ in their properties. Thus, in comparison with silicon, compound semiconductors have both advantages and disadvantages. For example, gallium arsenide (GaAs) has six times higher electron mobility than silicon, which allows faster operation; wider band gap, which allows op ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Silicide
Magnesium silicide, Mg2Si, is an inorganic compound consisting of magnesium and silicon. As-grown Mg2Si usually forms black crystals; they are semiconductors with n-type conductivity and have potential applications in thermoelectric generators. Crystal structure Mg2Si crystallizes in the antifluorite structure. In the face-centered cubic lattice Si centers occupy the corners and face-centered positions of the unit cell and Mg centers occupy eight tetrahedral sites in the interior of the unit cell. The coordination numbers of Si and Mg are eight and four, respectively. Synthesis It can be produced by heating silicon dioxide, SiO2, found in sand, with excess magnesium. The process first forms silicon metal and magnesium oxide, and, if an excess of SiO2 is used, then elemental silicon is formed: :2 Mg + SiO2 → 2 MgO + Si If an excess of Mg is present, Mg2Si is formed from the reaction of the remaining magnesium with the silicon: :2 Mg + Si → Mg2Si These reactions pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silver(I) Selenide
Silver selenide (Ag2Se) is the reaction product formed when selenium toning analog silver gelatine photo papers in photographic print toning. The selenium toner contains sodium selenite (Na2SeO3) as one of its active ingredients, which is the source of the selenide (Se2−) anion combining with the silver in the toning process. It is found in nature as the mineral naumannite, a comparatively rare silver mineral which has nevertheless become recognized as important silver compound in some low-sulfur silver ores from mines in Nevada and Idaho. Structure Silver selenide has two crystal phases on the bulk phase diagram. At lower temperatures, it has an orthorhombic structure, β-Ag2Se. This orthorhombic phase, stable at room temperature, is a narrow-gap semiconductor, with space group P212121. The exact size of the band gap has been given variously from 0.02 eV to 0.22 eV. There is also a high temperature cubic phase, α-Ag2Se., which it transforms into at temperatures above 130 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tin Selenide
Tin selenide, also known as stannous selenide, is an inorganic compound with the formula Sn Se. Tin(II) selenide is a typical layered metal chalcogenide as it includes a group 16 anion (Se2−) and an electropositive element (Sn2+), and is arranged in a layered structure. Tin(II) selenide is a narrow band-gap (IV-VI) semiconductor structurally analogous to black phosphorus. It has received considerable interest for applications including low-cost photovoltaics, and memory-switching devices. Because of its low thermal conductivity as well as reasonable electrical conductivity, tin selenide is one of the most efficient thermoelectric materials. Structure Tin(II) selenide (SnSe) crystallizes in the orthorhombic structure that derives from a distorted rock-salt structure. It is isomorphous to germanium selenide (GeSe). The unit cell encompasses two inverted layers. Each tin atom is covalently bonded to three neighboring selenium atoms, and each selenium atom is covalently bonded ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tin Telluride
Tin telluride is a compound of tin and tellurium (SnTe); is a IV-VI narrow band gap semiconductor and has direct band gap of 0.18 eV. It is often alloyed with lead to make lead tin telluride, which is used as an infrared detector material. Tin telluride normally forms p-type semiconductor (Extrinsic semiconductor) due to tin vacancies and is a low temperature superconductor. SnTe exists in three crystal phases. At Low temperatures, where the concentration of hole carriers is less than 1.5x1020 cm−3 , Tin Telluride exists in rhombohedral phase also known as α-SnTe. At room temperature and atmospheric pressure, Tin Telluride exists in NaCl-like cubic crystal phase, known as β-SnTe. While at 18 kbar pressure, β-SnTe transforms to γ-SnTe, orthorhombic phase, space group Pnma. This phase change is characterized by 11 percent increase in density and 360 percent increase in resistance for γ-SnTe. Tin telluride is a thermoelectric material. Theoretical studies imply that the n-t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuth Telluride
Bismuth telluride (Bi2Te3) is a gray powder that is a compound of bismuth and tellurium also known as bismuth(III) telluride. It is a semiconductor, which, when alloyed with antimony or selenium, is an efficient thermoelectric material for refrigeration or portable power generation. Bi2Te3 is a topological insulator, and thus exhibits thickness-dependent physical properties. Properties as a thermoelectric material Bismuth telluride is a narrow-gap layered semiconductor with a trigonal unit cell. The valence and conduction band structure can be described as a many-ellipsoidal model with 6 constant-energy ellipsoids that are centered on the reflection planes. Bi2Te3 cleaves easily along the trigonal axis due to Van der Waals bonding between neighboring tellurium atoms. Due to this, bismuth-telluride-based materials used for power generation or cooling applications must be polycrystalline. Furthermore, the Seebeck coefficient of bulk Bi2Te3 becomes compensated around room temperature, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
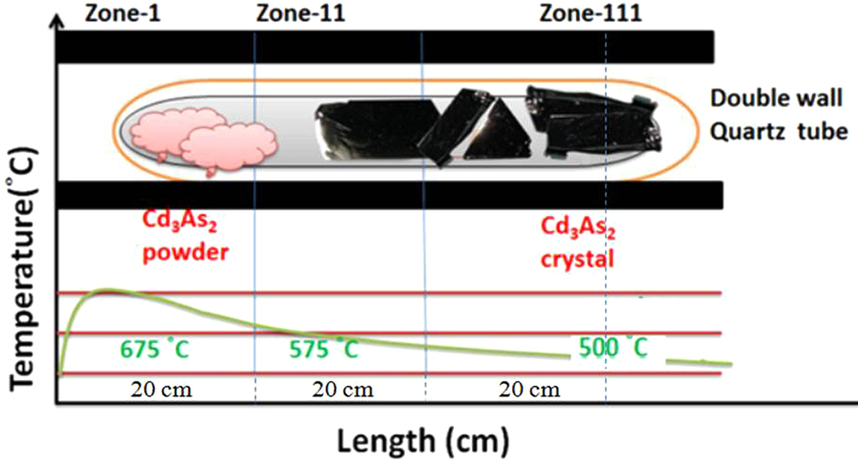
Cadmium Arsenide
Cadmium arsenide ( Cd3 As2) is an inorganic semimetal in the II-V family. It exhibits the Nernst effect. Properties Thermal Cd3As2 dissociates between 220 and 280 °C according to the reaction :2 Cd3As2(s) → 6 Cd(g) + As4(g) An energy barrier was found for the nonstoichiometric vaporization of arsenic due to the irregularity of the partial pressures with temperature. The range of the energy gap is from 0.5 to 0.6 eV. Cd3As2 melts at 716 °C and changes phase at 615 °C/ Phase transition Pure cadmium arsenide undergoes several phase transitions at high temperatures, making phases labeled α (stable), α’, α” (metastable), and β. At 593° the polymorphic transition α → β occurs. :α-Cd3As2 ↔ α’-Cd3As2 occurs at ~500 K. :α’-Cd3As2 ↔ α’’-Cd3As2 occurs at ~742 K and is a regular first order phase transition with marked hysteresis loop. :α”-Cd3As2 ↔ β-Cd3As2 occurs at 868 K. Single crystal x-ray diffraction was used to dete ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gallium Antimonide
Gallium antimonide (GaSb) is a semiconducting compound of gallium and antimony of the III-V family. It has a lattice constant of about 0.61 nm. It has a band gap of 0.67 eV. History The intermetallic compound GaSb was first prepared in 1926 by Victor Goldschmidt, who directly combined the elements under an inert gas atmosphere and reported on GaSb's lattice constant, which has since been revised. Goldschmidt also synthesized gallium phosphide and gallium arsenide. The Ga-Sb phase equilibria was investigated in 1955 by Koster and by Greenfield.Greenfield, I. G.; Smith, R. L., ''Trans. AIME'' 203, 351 (1955). Applications GaSb can be used for Infrared detectors, infrared LEDs and lasers and transistors, and thermophotovoltaic systems. See also * Aluminium antimonide * Indium antimonide * Gallium arsenide References External links properties listed at NSM Ioffe Institute. National Compound Semiconductor Roadmapat the Office of Naval Research The Office of Naval Research (ONR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indium Antimonide
Indium antimonide (InSb) is a crystalline compound made from the elements indium (In) and antimony (Sb). It is a narrow- gap semiconductor material from the III- V group used in infrared detectors, including thermal imaging cameras, FLIR systems, infrared homing missile guidance systems, and in infrared astronomy. The indium antimonide detectors are sensitive between 1–5 μm wavelengths. Indium antimonide was a very common detector in the old, single-detector mechanically scanned thermal imaging systems. Another application is as a terahertz radiation source as it is a strong photo-Dember emitter. History The intermetallic compound was first reported by Liu and Peretti in 1951, who gave its homogeneity range, structure type, and lattice constant. Polycrystalline ingots of InSb were prepared by Heinrich Welker in 1952, although they were not very pure by today's semiconductor standards. Welker was interested in systematically studying the semiconducting properties of t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
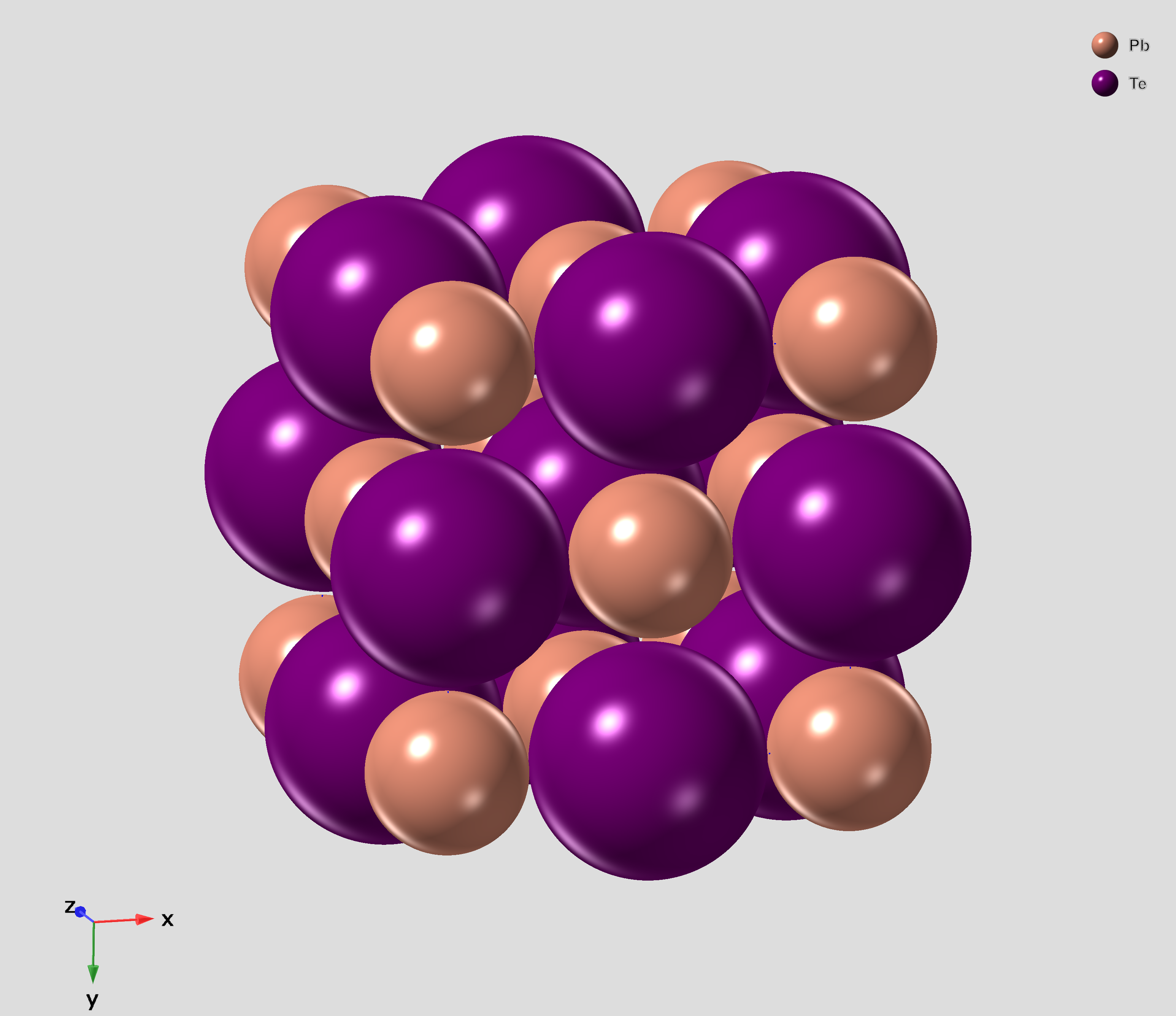
Lead Telluride
Lead telluride is a compound of lead and tellurium (PbTe). It crystallizes in the NaCl crystal structure with Pb atoms occupying the cation and Te forming the anionic lattice. It is a narrow gap semiconductor with a band gap of 0.32 eV. It occurs naturally as the mineral altaite. Properties * Dielectric constant ~1000. * Electron Effective mass ~ 0.01 ''m''e * Hole mobility, μp = 600 cm2 V−1 s−1 (0 K); 4000 cm2 V−1 s−1 (300 K) Applications PbTe has proven to be a very important intermediate thermoelectric material. The performance of thermoelectric materials can be evaluated by the figure of merit, ZT=S^2\sigma T/\kappa, in which S is the Seebeck coefficient, \sigma is the electrical conductivity and \kappa is the thermal conductivity. In order to improve the thermoelectric performance of materials, the power factor (S^2\sigma) needs to be maximized and the thermal conductivity needs to be minimized. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |