|
Nubian Sandstone Aquifer System
The Nubian Sandstone Aquifer System (NSAS) is the world's largest known fossil water aquifer system. It is located underground in the Eastern end of the Sahara desert and spans the political boundaries of four countries in north-eastern Africa. NSAS covers a land area spanning just over two million km2, including north-western Sudan, north-eastern Chad, south-eastern Libya, and most of Egypt. Containing an estimated 150,000 km3 of groundwater, the significance of the NSAS as a potential water resource for future development programs in these countries is extraordinary. The Great Man-Made River (GMMR) project in Libya makes use of the system, extracting substantial amounts of water from this aquifer, removing an estimated 2.4 km3 of fresh water for consumption and agriculture per year. Characteristics Since 2001, the Nubian Sandstone aquifer situated between the Toshka and Abu Simbel areas of Egypt has undergone intensive drilling and development as part of a land ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
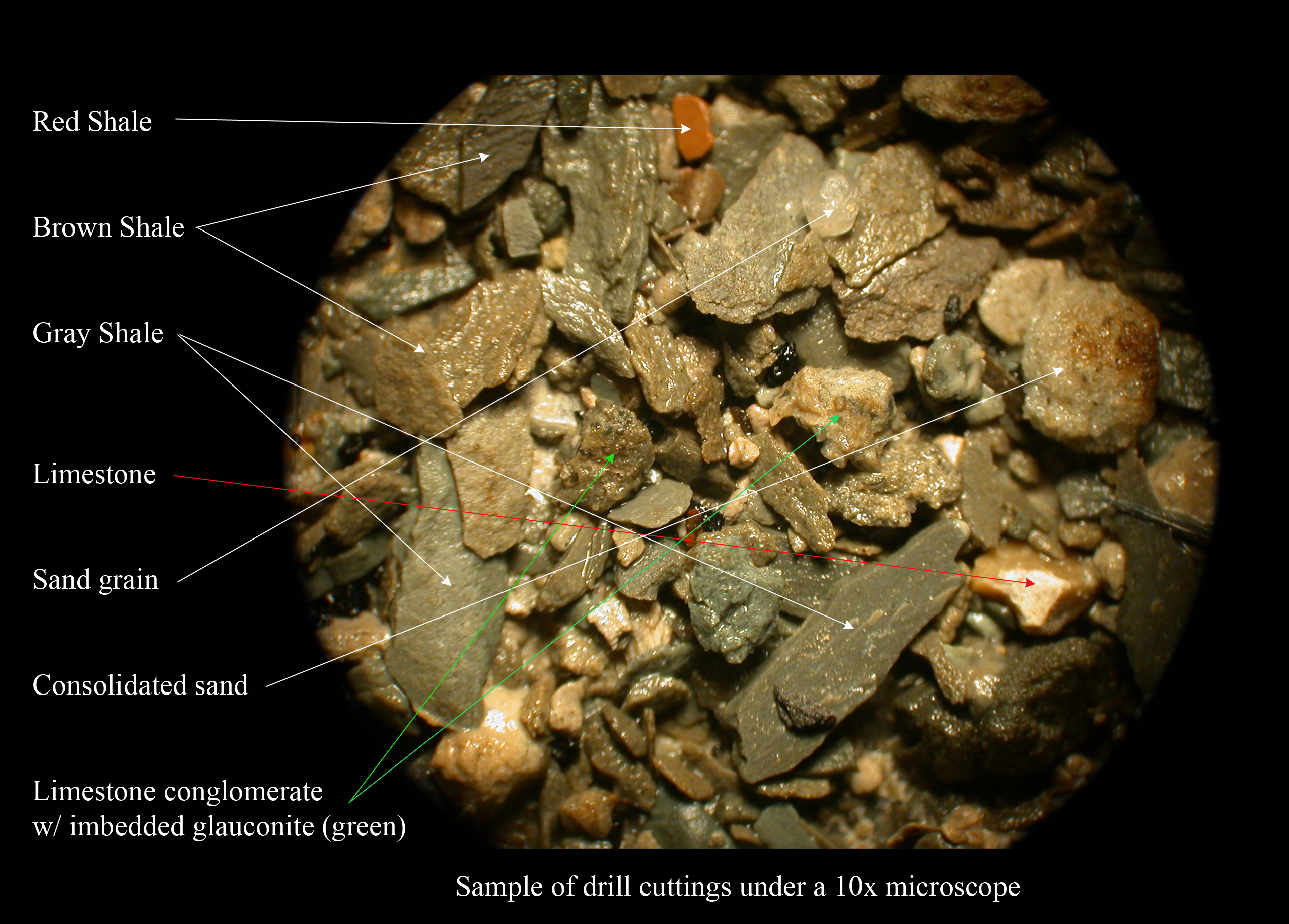
Lithological
The lithology of a Rock (geology), rock unit is a description of its physical characteristics visible at outcrop, in hand or core sample, core samples, or with low magnification microscopy. Physical characteristics include colour, texture, grain size, and composition. Lithology may refer to either a detailed description of these characteristics, or a summary of the gross physical character of a rock. Examples of lithologies in the second sense include sandstone, slate, basalt, or limestone. Lithology is the basis of subdividing rock sequences into individual Lithostratigraphy, lithostratigraphic units for the purposes of Geologic map, mapping and correlation between areas. In certain applications, such as Geotechnical investigation, site investigations, lithology is described using a standard terminology such as in the European geotechnical standard Eurocode 7: Geotechnical design, Eurocode 7. Rock type The naming of a lithology is based on the List of rock types, rock type. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element with the symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table) it occurs naturally only in combination with other elements and it almost always has an oxidation state of +2. It reacts readily with air to form a thin passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three helium nuclei to a carbon nucleus. When such stars explode as supernovas, much of the magnesium is expelled into the interstellar medium where it ma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' "lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element with the symbol Na (from Latin ''natrium'') and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature, and must be prepared from compounds. Sodium is the sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been leached by the action of water from the Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in soap manufacture, and sodium chloride (edible salt) is a de-icing agent and a nutrient for animals including h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Parts Per Million
In science and engineering, the parts-per notation is a set of pseudo-units to describe small values of miscellaneous dimensionless quantities, e.g. mole fraction or mass fraction. Since these fractions are quantity-per-quantity measures, they are pure numbers with no associated units of measurement. Commonly used are parts-per-million (ppm, ), parts-per-billion (ppb, ), parts-per-trillion (ppt, ) and parts-per-quadrillion (ppq, ). This notation is not part of the International System of Units (SI) system and its meaning is ambiguous. Overview Parts-per notation is often used describing dilute solutions in chemistry, for instance, the relative abundance of dissolved minerals or pollutants in water. The quantity "1 ppm" can be used for a mass fraction if a water-borne pollutant is present at one-millionth of a gram per gram of sample solution. When working with aqueous solutions, it is common to assume that the density of water is 1.00 g/mL. Therefore, it is common to equat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
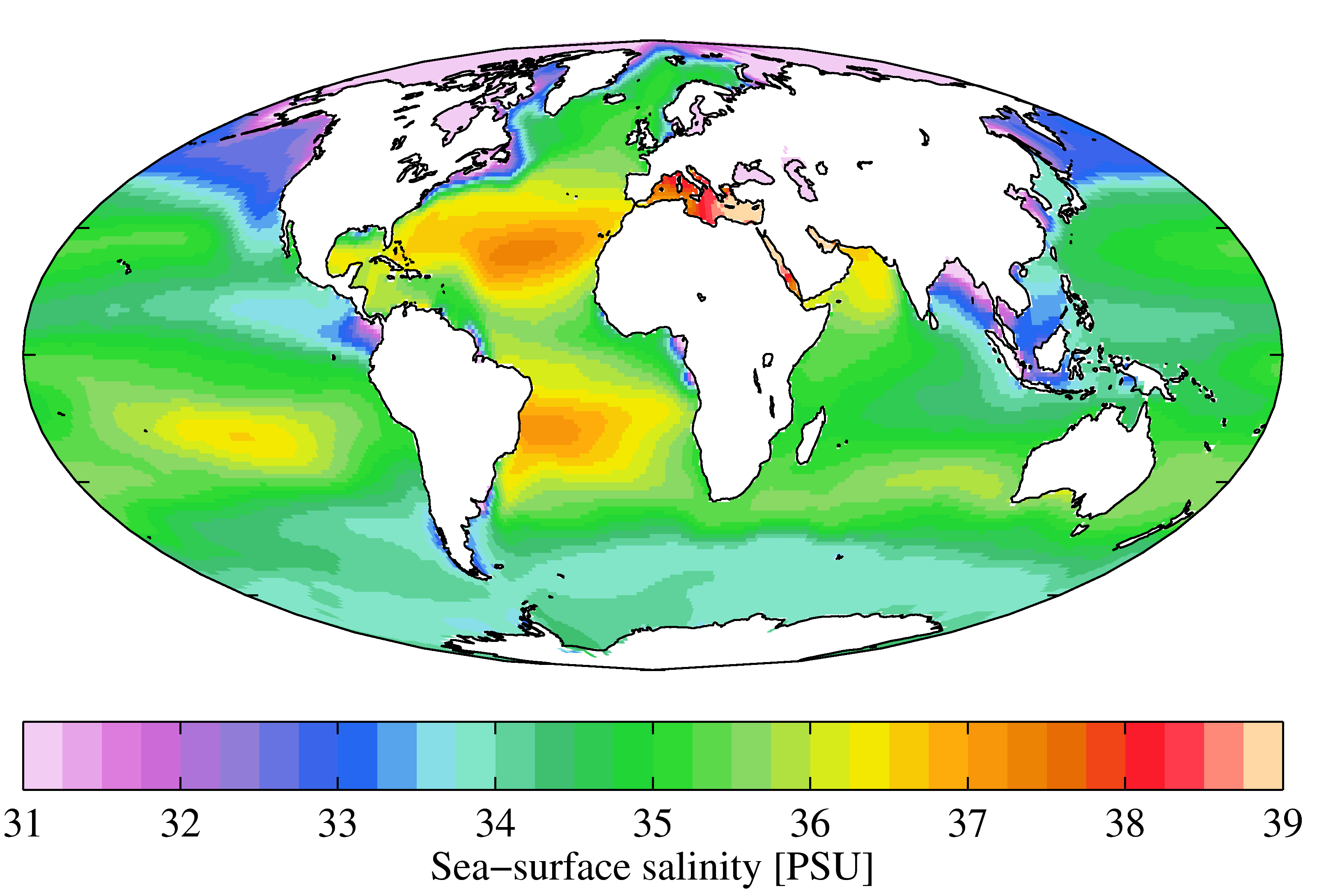
Salinity
Salinity () is the saltiness or amount of salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal to ‰). Salinity is an important factor in determining many aspects of the chemistry of natural waters and of biological processes within it, and is a thermodynamic state variable that, along with temperature and pressure, governs physical characteristics like the density and heat capacity of the water. A contour line of constant salinity is called an ''isohaline'', or sometimes ''isohale''. Definitions Salinity in rivers, lakes, and the ocean is conceptually simple, but technically challenging to define and measure precisely. Conceptually the salinity is the quantity of dissolved salt content of the water. Salts are compounds like sodium chloride, magnesium sulfate, potassium nitrate, and sodium bicarbonate which dissolve into ions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interbedding
In geology, interbedding occurs when beds (layers of rock) of a particular lithology lie between or alternate with beds of a different lithology. For example, sedimentary rocks may be interbedded if there were sea level variations in their sedimentary depositional environment. Intercalation is a special case of interbedding where a layer is variably inserted into an already existing sequence; or where two separate depositional environments in close spatial proximity migrate alternately across the contact. While interbedding has layers that are horizontally flat (or aligned with the angle of the entire stratum), intercalated rock on the other hand has slanted layers that streak through each other (even when it aligns with the stratum). For example intercalated conglomerate and sandstone looks like ripples of different material networked through each other somewhat off the horizontal, as the beds are deposited in a gradient. This is likely due to differing fluvial conditions and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shale
Shale is a fine-grained, clastic sedimentary rock formed from mud that is a mix of flakes of clay minerals (hydrous aluminium phyllosilicates, e.g. kaolin, Al2 Si2 O5( OH)4) and tiny fragments (silt-sized particles) of other minerals, especially quartz and calcite.Blatt, Harvey and Robert J. Tracy (1996) ''Petrology: Igneous, Sedimentary and Metamorphic'', 2nd ed., Freeman, pp. 281–292 Shale is characterized by its tendency to split into thin layers ( laminae) less than one centimeter in thickness. This property is called '' fissility''. Shale is the most common sedimentary rock. The term ''shale'' is sometimes applied more broadly, as essentially a synonym for mudrock, rather than in the more narrow sense of clay-rich fissile mudrock. Texture Shale typically exhibits varying degrees of fissility. Because of the parallel orientation of clay mineral flakes in shale, it breaks into thin layers, often splintery and usually parallel to the otherwise indistinguishable beddin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sandstone
Sandstone is a clastic sedimentary rock composed mainly of sand-sized (0.0625 to 2 mm) silicate grains. Sandstones comprise about 20–25% of all sedimentary rocks. Most sandstone is composed of quartz or feldspar (both silicates) because they are the most resistant minerals to weathering processes at the Earth's surface. Like uncemented sand, sandstone may be any color due to impurities within the minerals, but the most common colors are tan, brown, yellow, red, grey, pink, white, and black. Since sandstone beds often form highly visible cliffs and other topographic features, certain colors of sandstone have been strongly identified with certain regions. Rock formations that are primarily composed of sandstone usually allow the percolation of water and other fluids and are porous enough to store large quantities, making them valuable aquifers and petroleum reservoirs. Quartz-bearing sandstone can be changed into quartzite through metamorphism, usually related to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron Oxide
Iron oxides are chemical compounds composed of iron and oxygen. Several iron oxides are recognized. All are black magnetic solids. Often they are non-stoichiometric. Oxyhydroxides are a related class of compounds, perhaps the best known of which is rust. Iron oxides and oxyhydroxides are widespread in nature and play an important role in many geological and biological processes. They are used as iron ores, pigments, catalysts, and in thermite, and occur in hemoglobin. Iron oxides are inexpensive and durable pigments in paints, coatings and colored concretes. Colors commonly available are in the "earthy" end of the yellow/orange/red/brown/black range. When used as a food coloring, it has E number E172. Stoichiometries Iron oxides feature as ferrous ( Fe(II)) or ferric ( Fe(III)) or both. They adopt octahedral or tetrahedral coordination geometry. Only a few oxides are significant at the earth's surface, particularly wüstite, magnetite, and hematite. * Oxides of FeII ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |





.jpg)

Saunders_Quarry-1.jpg)
