Salinity on:
[Wikipedia]
[Google]
[Amazon]
 Salinity () is the saltiness or amount of
Salinity () is the saltiness or amount of
Background papers and supporting data on the Practical Salinity Scale 1978
''Tech. Pap. Mar. Sci.'', 37 Salinities measured using PSS-78 do not have units. The suffix psu or PSU (denoting ''practical salinity unit'') is sometimes added to PSS-78 measurement values. The addition of PSU as a unit after the value is "formally incorrect and strongly discouraged". In 2010 a new standard for the properties of seawater called the ''thermodynamic equation of seawater 2010'' (
MIT page of seawater properties, with Matlab, EES and Excel VBA library routinesEquations and algorithms to calculate fundamental properties of sea water.History of the salinity determinationPractical Salinity Scale 1978.Salinity calculatorLewis, E. L. 1982. The practical salinity scale of 1978 and its antecedents. Marine Geodesy. 5(4):350–357.Equations and algorithms to calculate salinity of inland waters
{{Authority control Chemical oceanography Aquatic ecology Oceanography Coastal geography Water quality indicators Articles containing video clips Salts
 Salinity () is the saltiness or amount of
Salinity () is the saltiness or amount of salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quanti ...
dissolved in a body of water
Water (chemical formula ) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as ...
, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal to ‰).
Salinity is an important factor in determining many aspects of the chemistry of natural waters and of biological
Biology is the scientific study of life. It is a natural science with a broad scope but has several unifying themes that tie it together as a single, coherent field. For instance, all organisms are made up of cells that process hereditary ...
processes within it, and is a thermodynamic state variable that, along with temperature
Temperature is a physical quantity that expresses quantitatively the perceptions of hotness and coldness. Temperature is measured with a thermometer.
Thermometers are calibrated in various temperature scales that historically have relied on ...
and pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country a ...
, governs physical characteristics like the density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematicall ...
and heat capacity of the water.
A contour line of constant salinity is called an ''isohaline'', or sometimes ''isohale''.
Definitions
Salinity in rivers, lakes, and the ocean is conceptually simple, but technically challenging to define and measure precisely. Conceptually the salinity is the quantity of dissolved salt content of the water. Salts are compounds likesodium chloride
Sodium chloride , commonly known as salt (although sea salt also contains other chemical salts), is an ionic compound with the chemical formula NaCl, representing a 1:1 ratio of sodium and chloride ions. With molar masses of 22.99 and 35 ...
, magnesium sulfate, potassium nitrate, and sodium bicarbonate which dissolve into ions. The concentration of dissolved chloride ions is sometimes referred to as chlorinity. Operationally, dissolved matter is defined as that which can pass through a very fine filter (historically a filter with a pore size of 0.45 μm, but nowadays usually 0.2 μm). Salinity can be expressed in the form of a mass fraction, i.e. the mass of the dissolved material in a unit mass of solution.
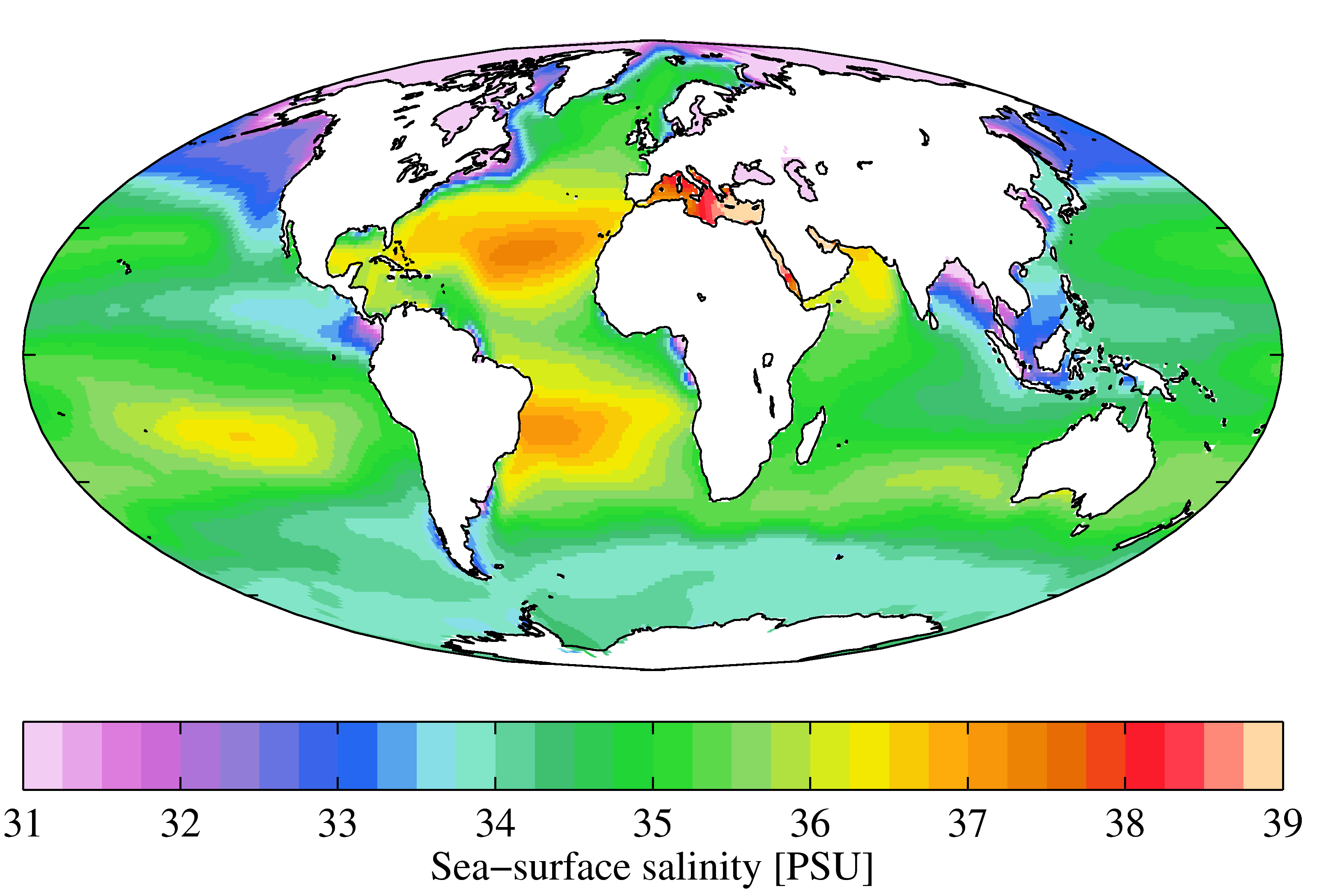
Seawater typically has a mass salinity of around 35 g/kg, although lower values are typical near coasts where rivers enter the ocean. Rivers and lakes can have a wide range of salinities, from less than 0.01 g/kg to a few g/kg, although there are many places where higher salinities are found. The Dead Sea
The Dead Sea ( he, יַם הַמֶּלַח, ''Yam hamMelaḥ''; ar, اَلْبَحْرُ الْمَيْتُ, ''Āl-Baḥrū l-Maytū''), also known by other names, is a salt lake bordered by Jordan to the east and Israel and the West Bank ...
has a salinity of more than 200 g/kg. Precipitation typically has a TDS of 20 mg/kg or less.
Whatever pore size is used in the definition, the resulting salinity value of a given sample of natural water will not vary by more than a few percent (%). Physical oceanographers working in the abyssal ocean, however, are often concerned with precision and intercomparability of measurements by different researchers, at different times, to almost five significant digits
Significant figures (also known as the significant digits, ''precision'' or ''resolution'') of a number in positional notation are digits in the number that are reliable and necessary to indicate the quantity of something.
If a number expres ...
. A bottled seawater product known as IAPSO Standard Seawater is used by oceanographers to standardize their measurements with enough precision to meet this requirement.
Composition
Measurement and definition difficulties arise because natural waters contain a complex mixture of many different elements from different sources (not all from dissolved salts) in different molecular forms. The chemical properties of some of these forms depend on temperature and pressure. Many of these forms are difficult to measure with high accuracy, and in any case complete chemical analysis is not practical when analyzing multiple samples. Different practical definitions of salinity result from different attempts to account for these problems, to different levels of precision, while still remaining reasonably easy to use. For practical reasons salinity is usually related to the sum of masses of a subset of these dissolved chemical constituents (so-called ''solution salinity''), rather than to the unknown mass of salts that gave rise to this composition (an exception is when artificial seawater is created). For many purposes this sum can be limited to a set of eight major ions in natural waters, although for seawater at highest precision an additional seven minor ions are also included. The major ions dominate the inorganic composition of most (but by no means all) natural waters. Exceptions include some pit lakes and waters from some hydrothermal springs. The concentrations of dissolved gases likeoxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
and nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seve ...
are not usually included in descriptions of salinity. However, carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
gas, which when dissolved is partially converted into carbonates and bicarbonates, is often included. Silicon in the form of silicic acid, which usually appears as a neutral molecule in the pH range of most natural waters, may also be included for some purposes (e.g., when salinity/density relationships are being investigated).
Seawater
The term 'salinity' is, for oceanographers, usually associated with one of a set of specific measurement techniques. As the dominant techniques evolve, so do different descriptions of salinity. Salinities were largely measured using titration-based techniques before the 1980s. Titration with silver nitrate could be used to determine the concentration of halide ions (mainlychlorine
Chlorine is a chemical element with the symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is ...
and bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simil ...
) to give a chlorinity
Salinity () is the saltiness or amount of salt dissolved in a body of water, called saline water (see also soil salinity). It is usually measured in g/L or g/kg (grams of salt per liter/kilogram of water; the latter is dimensionless and equal ...
. The chlorinity was then multiplied by a factor to account for all other constituents. The resulting 'Knudsen salinities' are expressed in units of parts per thousand (ppt or ‰).
The use of electrical conductivity measurements to estimate the ionic content of seawater led to the development of the scale called the ''practical salinity scale 1978'' (PSS-78).Unesco (1981). The Practical Salinity Scale 1978 and the International Equation of State of Seawater 1980. ''Tech. Pap. Mar. Sci.'', 36Unesco (1981)Background papers and supporting data on the Practical Salinity Scale 1978
''Tech. Pap. Mar. Sci.'', 37 Salinities measured using PSS-78 do not have units. The suffix psu or PSU (denoting ''practical salinity unit'') is sometimes added to PSS-78 measurement values. The addition of PSU as a unit after the value is "formally incorrect and strongly discouraged". In 2010 a new standard for the properties of seawater called the ''thermodynamic equation of seawater 2010'' (
TEOS-10
TEOS-10 (Thermodynamic Equation of Seawater - 2010) is the international standard for the use and calculation of the thermodynamic properties of seawater, humid air and ice. It supersedes the former standard EOS-80 (Equation of State of Seawater 1 ...
) was introduced, advocating absolute salinity as a replacement for practical salinity, and conservative temperature
Conservative temperature (\Theta) is a thermodynamic property of seawater. It is derived from the potential enthalpy and is recommended under the TEOS-10 standard (Thermodynamic Equation of Seawater - 2010) as a replacement for potential temper ...
as a replacement for potential temperature
The potential temperature of a parcel of fluid at pressure P is the temperature that the parcel would attain if adiabatically brought to a standard reference pressure P_, usually . The potential temperature is denoted \theta and, for a gas well-a ...
. This standard includes a new scale called the ''reference composition salinity scale''. Absolute salinities on this scale are expressed as a mass fraction, in grams per kilogram of solution. Salinities on this scale are determined by combining electrical conductivity measurements with other information that can account for regional changes in the composition of seawater. They can also be determined by making direct density measurements.
A sample of seawater from most locations with a chlorinity of 19.37 ppt will have a Knudsen salinity of 35.00 ppt, a PSS-78 practical salinity of about 35.0, and a TEOS-10 absolute salinity of about 35.2 g/kg. The electrical conductivity of this water at a temperature of 15 °C is 42.9 mS/cm.
On the global scale, it is extremely likely that human-caused climate change has contributed to observed surface and subsurface salinity changes since the 1950s, and projections of surface salinity changes throughout the 21st century indicate that fresh ocean regions will continue to get fresher and salty regions will continue to get saltier.
Lakes and rivers
Limnologist
Limnology ( ; from Greek λίμνη, ''limne'', "lake" and λόγος, ''logos'', "knowledge") is the study of inland aquatic ecosystems.
The study of limnology includes aspects of the biological, chemical, physical, and geological characteris ...
s and chemists often define salinity in terms of mass of salt per unit volume, expressed in units of mg/L or g/L. It is implied, although often not stated, that this value applies accurately only at some reference temperature because solution volume varies with temperature. Values presented in this way are typically accurate to the order of 1%. Limnologists also use electrical conductivity, or "reference conductivity", as a proxy for salinity. This measurement may be corrected for temperature effects, and is usually expressed in units of μS/cm.
A river or lake water with a salinity of around 70 mg/L will typically have a specific conductivity at 25 °C of between 80 and 130 μS/cm. The actual ratio depends on the ions present. The actual conductivity usually changes by about 2% per degree Celsius, so the measured conductivity at 5 °C might only be in the range of 50–80 μS/cm.
Direct density measurements are also used to estimate salinities, particularly in highly saline lakes. Sometimes density at a specific temperature is used as a proxy for salinity. At other times an empirical salinity/density relationship developed for a particular body of water is used to estimate the salinity of samples from a measured density.
Classification of water bodies based upon salinity
Marine waters are those of the ocean, another term for which is ''euhaline seas''. The salinity of euhaline seas is 30 to 35 ‰. ''Brackish seas'' or waters have salinity in the range of 0.5 to 29 ‰ and ''metahaline seas'' from 36 to 40 ‰. These waters are all regarded as ''thalassic'' because their salinity is derived from the ocean and defined as ''homoiohaline'' if salinity does not vary much over time (essentially constant). The table on the right, modified from Por (1972), follows the "Venice system" (1959). In contrast to homoiohaline environments are certain ''poikilohaline'' environments (which may also be ''thalassic'') in which the salinity variation is biologically significant. ''Poikilohaline'' water salinities may range anywhere from 0.5 to greater than 300 ‰. The important characteristic is that these waters tend to vary in salinity over some biologically meaningful range seasonally or on some other roughly comparable time scale. Put simply, these are bodies of water with quite variable salinity. Highly saline water, from which salts crystallize (or are about to), is referred to asbrine
Brine is a high-concentration Solution (chemistry), solution of salt (NaCl) in water (H2O). In diverse contexts, ''brine'' may refer to the salt solutions ranging from about 3.5% (a typical concentration of seawater, on the lower end of that of ...
.
Environmental considerations
Salinity is an ecological factor of considerable importance, influencing the types of organisms that live in a body of water. As well, salinity influences the kinds ofplant
Plants are predominantly Photosynthesis, photosynthetic eukaryotes of the Kingdom (biology), kingdom Plantae. Historically, the plant kingdom encompassed all living things that were not animals, and included algae and fungi; however, all curr ...
s that will grow either in a water body, or on land fed by a water (or by a groundwater
Groundwater is the water present beneath Earth's surface in rock and soil pore spaces and in the fractures of rock formations. About 30 percent of all readily available freshwater in the world is groundwater. A unit of rock or an unconsolidat ...
). A plant adapted to saline conditions is called a halophyte. A halophyte which is tolerant to residual sodium carbonate salinity are called glasswort or saltwort or barilla plants. Organisms (mostly bacteria) that can live in very salty conditions are classified as extremophile
An extremophile (from Latin ' meaning "extreme" and Greek ' () meaning "love") is an organism that is able to live (or in some cases thrive) in extreme environments, i.e. environments that make survival challenging such as due to extreme tempe ...
s, or halophiles specifically. An organism that can withstand a wide range of salinities is euryhaline.
Salts are expensive to remove from water, and salt content is an important factor in water use, factoring into potability and suitability for irrigation
Irrigation (also referred to as watering) is the practice of applying controlled amounts of water to land to help grow crops, landscape plants, and lawns. Irrigation has been a key aspect of agriculture for over 5,000 years and has been dev ...
. Increases in salinity have been observed in lakes and rivers in the United States, due to common road salt and other salt de-icers in runoff.
The degree of salinity in oceans is a driver of the world's ocean circulation, where density changes due to both salinity changes and temperature changes at the surface of the ocean produce changes in buoyancy, which cause the sinking and rising of water masses. Changes in the salinity of the oceans are thought to contribute to global changes in carbon dioxide as more saline waters are less soluble to carbon dioxide. In addition, during glacial periods, the hydrography is such that a possible cause of reduced circulation is the production of stratified oceans. In such cases, it is more difficult to subduct water through the thermohaline circulation.
Not only is salinity a driver of ocean circulation, but changes in ocean circulation also affect salinity, particularly in the subpolar North Atlantic where from 1990 to 2010 increased contributions of Greenland meltwater were counteracted by increased northward transport of salty Atlantic waters. However, North Atlantic waters have become fresher since the mid-2010s due to increased Greenland meltwater flux.
See also
*Desalination for economic purposes ** Desalination of water **Desalination of soil: soil salinity control ** Sodium adsorption ratio * Measuring salinity ** Salinometer * Salinity by biologic context ** In organisms generally, with particular emphasis on human health *** Electrolytes *** Fluid balance *** Hypernatremia *** Hyponatremia *** Salt poisoning ** In plants *** ''Arabidopsis thaliana'' responses to salinity ** In fish *** Stenohaline fish *** Euryhaline fish *Salinity by geologic context ** Fresh water **Seawater
Seawater, or salt water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has appr ...
** Soil salinity
** Thermohaline circulation
**Paleosalinity
Paleosalinity (or palaeosalinity) is the salinity of the global ocean or of an ocean basin at a point in geological history.
Importance
From Bjerrum plots, it is found that a decrease in the salinity of an aqueous fluid will act to increase th ...
** CORA dataset data on salinity of global oceans
*General cases of solute concentration
** Osmotic concentration
** Tonicity
References
Further reading
*MIT page of seawater properties, with Matlab, EES and Excel VBA library routines
{{Authority control Chemical oceanography Aquatic ecology Oceanography Coastal geography Water quality indicators Articles containing video clips Salts