|
Arabidopsis Thaliana Responses To Salinity
As a model organism, the '' Arabidopsis thaliana'' response to salinity is studied to aid understanding of other more economically important crops. High concentration of salt in the soil has negative effects on plants. For example, it reduces the yield that crop plants can produce in 7% of the land. On the other side, some plants show adaptations to changes in soil salinity, in that the plant's exposure to salt initiates certain mechanisms for cell osmotic regulation and causes changes in this plant's water obtaining and loss behaviors. One of such plants is the model plant ''Arabidopsis thaliana'', a member of the family Brassicaceae. ''Arabidopsis thaliana'' is native to Eurasia and was introduced to some parts of North America. It grows in rocky, sandy and disturbed terrains. It has been found in many studies that ''Arabidopsis thaliana'' showed enhanced Na+ and H+ extrusion from their cells after exposure to high salinity. Part of ''Arabidopsis''’ range might have included ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arabidopsis Thaliana
''Arabidopsis thaliana'', the thale cress, mouse-ear cress or arabidopsis, is a small flowering plant native to Eurasia and Africa. ''A. thaliana'' is considered a weed; it is found along the shoulders of roads and in disturbed land. A winter annual with a relatively short lifecycle, ''A. thaliana'' is a popular model organism in plant biology and genetics. For a complex multicellular eukaryote, ''A. thaliana'' has a relatively small genome around 135 megabase pairs. It was the first plant to have its genome sequenced, and is a popular tool for understanding the molecular biology of many plant traits, including flower development and light sensing. Description ''Arabidopsis thaliana'' is an annual (rarely biennial) plant, usually growing to 20–25 cm tall. The leaves form a rosette at the base of the plant, with a few leaves also on the flowering stem. The basal leaves are green to slightly purplish in color, 1.5–5 cm long, and 2–10 mm broad, wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
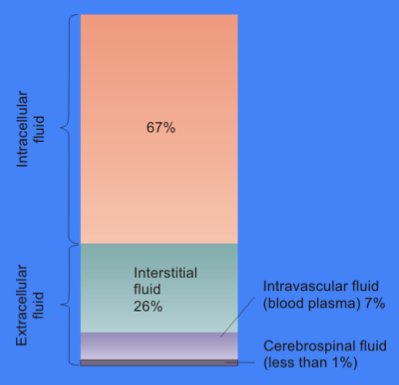
Cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into many compartments. In the eukaryotic cell, the cytosol is surrounded by the cell membrane and is part of the cytoplasm, which also comprises the mitochondria, plastids, and other organelles (but not their internal fluids and structures); the cell nucleus is separate. The cytosol is thus a liquid matrix around the organelles. In prokaryotes, most of the chemical reactions of metabolism take place in the cytosol, while a few take place in membranes or in the periplasmic space. In eukaryotes, while many metabolic pathways still occur in the cytosol, others take place within organelles. The cytosol is a complex mixture of substances dissolved in water. Although water forms the large majority of the cytosol, its structure and prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-gal Dish
X-gal (also abbreviated BCIG for 5-bromo-4-chloro-3-indolyl-β--galactopyranoside) is an organic compound consisting of galactose linked to a substituted indole. The compound was synthesized by Jerome Horwitz and collaborators in 1964. The formal chemical name is often shortened to less accurate but also less cumbersome phrases such as bromochloroindoxyl galactoside. The X from indoxyl may be the source of the X in the X-gal contraction. X-gal is often used in molecular biology to test for the presence of an enzyme, β-galactosidase, in the place of its usual target, a β-galactoside. It is also used to detect activity of this enzyme in histochemistry and bacteriology. X-gal is one of many indoxyl glycosides and esters that yield insoluble blue compounds similar to indigo dye as a result of enzyme-catalyzed hydrolysis. Uses X-gal is an analog of lactose, and therefore may be hydrolyzed by the β-galactosidase enzyme which cleaves the β- glycosidic bond in -lactose. X-gal, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphatidic Acid
Phosphatidic acids are anionic phospholipids important to cell signaling and direct activation of lipid-gated ion channels. Hydrolysis of phosphatidic acid gives rise to one molecule each of glycerol and phosphoric acid and two molecules of fatty acids. They constitute about 0.25% of phospholipids in the bilayer. Structure Phosphatidic acid consists of a glycerol backbone, with, in general, a saturated fatty acid bonded to carbon-1, an unsaturated fatty acid bonded to carbon-2, and a phosphate group bonded to carbon-3. Formation and degradation Besides de novo synthesis, PA can be formed in three ways: * By phospholipase D (PLD), via the hydrolysis of the P-O bond of phosphatidylcholine (PC) to produce PA and choline. * By the phosphorylation of diacylglycerol (DAG) by DAG kinase (DAGK). * By the acylation of lysophosphatidic acid by lysoPA-acyltransferase (LPAAT); this is the most common pathway.Devlin, T. M. 2004. ''Bioquímica'', 4ª edición. Reverté, Barcelona. The glycer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PLD1
Phospholipase D1 (PLD1) is an enzyme that in humans is encoded by the ''PLD1'' gene, though analogues are found in plants, fungi, prokaryotes, and even viruses. History The possibility of PLD1 was first mentioned in 1947 by authors Hanahan and Chaikoff at Berkeley when describing a carrot enzyme that could " plitcholine from phospholipids." PLD was first derived in mammals in 1975 by Saito and Kanfer, who noted its activity in rats. PLD was first cloned from HeLa cell cDNA in 1995, while mammalian PLD1 was first cloned from a rat in 1997. Function Phosphatidylcholine (PC)-specific phospholipases D (PLDs) catalyze the hydrolysis of PC to produce phosphatidic acid (PA) and choline. A range of agonists acting through G protein-coupled receptors and receptor tyrosine kinases stimulate this hydrolysis. PC-specific PLD activity has been implicated in numerous cellular pathways, including membrane trafficking, signal transduction, platelet coagulation, mitosis, apoptosis, and the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antiporter
An antiporter (also called exchanger or counter-transporter) is a cotransporter and integral membrane protein involved in secondary active transport of two or more different molecules or ions across a phospholipid membrane such as the plasma membrane in opposite directions, one into the cell and one out of the cell. Na+/H+ antiporters have been reviewed. In secondary active transport, one species of solute moves along its electrochemical gradient, allowing a different species to move against its own electrochemical gradient. This movement is in contrast to primary active transport, in which all solutes are moved against their concentration gradients, fueled by ATP. Transport may involve one or more of each type of solute. For example, the Na+/Ca2+ exchanger, found in the plasma membrane of many cells, moves three sodium ions in one direction, and one calcium ion in the other. Role in Homeostatic Mechanisms Na+/H+ Antiporters Antiporters, such as Na+/H+ antiporter pr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasma Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorylated
In chemistry, phosphorylation is the attachment of a phosphate group to a molecule or an ion. This process and its inverse, dephosphorylation, are common in biology and could be driven by natural selection. Text was copied from this source, which is available under a Creative Commons Attribution 4.0 International License. Protein phosphorylation often activates (or deactivates) many enzymes. Glucose Phosphorylation of sugars is often the first stage in their catabolism. Phosphorylation allows cells to accumulate sugars because the phosphate group prevents the molecules from diffusing back across their transporter. Phosphorylation of glucose is a key reaction in sugar metabolism. The chemical equation for the conversion of D-glucose to D-glucose-6-phosphate in the first step of glycolysis is given by :D-glucose + ATP → D-glucose-6-phosphate + ADP :ΔG° = −16.7 kJ/mol (° indicates measurement at standard condition) Hepatic cells are freely permeable to glucose, and the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kinase
In biochemistry, a kinase () is an enzyme that catalysis, catalyzes the transfer of phosphate groups from High-energy phosphate, high-energy, phosphate-donating molecules to specific Substrate (biochemistry), substrates. This process is known as phosphorylation, where the high-energy adenosine triphosphate, ATP molecule donates a phosphate group to the substrate (biology), substrate molecule. This transesterification produces a phosphorylated substrate and Adenosine diphosphate, ADP. Conversely, it is referred to as dephosphorylation when the phosphorylated substrate donates a phosphate group and adenosine diphosphate, ADP gains a phosphate group (producing a dephosphorylated substrate and the high energy molecule of ATP). These two processes, phosphorylation and dephosphorylation, occur four times during glycolysis. Kinases are part of the larger family of phosphotransferases. Kinases should not be confused with phosphorylases, which catalyze the addition of inorganic phosphate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium-binding Protein
Calcium-binding proteins are proteins that participate in calcium cell signalling pathways by binding to Ca2+, the calcium ion that plays an important role in many cellular processes. Calcium-binding proteins have specific domains that bind to calcium and are known to be heterogeneous. One of the functions of calcium binding proteins is to regulate the amount of free (unbound) Ca2+ in the cytosol of the cell. The cellular regulation of calcium is known as calcium homeostasis. Types Many different calcium-binding proteins exist, with different cellular and tissue distribution and involvement in specific functions. Calcium binding proteins also serve an important physiological role for cells. The most ubiquitous Ca2+-sensing protein, found in all eukaryotic organisms including yeasts, is calmodulin. Intracellular storage and release of Ca2+ from the sarcoplasmic reticulum is associated with the high-capacity, low-affinity calcium-binding protein calsequestrin. Calretinin is anoth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |