|
Norbornene
Norbornene or norbornylene or norcamphene is a highly strained bridged cyclic hydrocarbon. It is a white solid with a pungent sour odor. The molecule consists of a cyclohexene ring with a methylene bridge between carbons 1 and 4. The molecule carries a double bond which induces significant ring strain and significant reactivity. Production Norbornene is made by a Diels–Alder reaction of cyclopentadiene and ethylene. Many substituted norbornenes can be prepared similarly. Related bicyclic compounds are norbornadiene, which has the same carbon skeleton but with two double bonds, and norbornane which is prepared by hydrogenation of norbornene. Reactions Norbornene undergoes an acid-catalyzed hydration reaction to form norborneol. This reaction was of great interest in the elucidation of the non-classical carbonion controversy. Norbornene is used in the Catellani reaction and in norbornene-mediated ''meta''-C−H activation. Certain substituted norbornenes undergo unus ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylidene Norbornene
Ethylidene norbornene (ENB) is an organic compound that consists of an ethylidene (CH3C(H)=) group attached to norbornene. It is a colorless liquid. The molecule consists of two sites of unsaturation, one of which participates in the copolymerization and the second of which (the ethylidene) undergoes vulcanization. It is a monomer in the production of the commercial polymer EPDM. The compound consists of E- and Z-stereoisomers, but the mixtures are not separated. It is prepared by isomerization of vinyl norbornene, which in turn is obtained by the Diels-Alder reaction of butadiene and cyclopentadiene Cyclopentadiene is an organic compound with the formula C5H6.LeRoy H. Scharpen and Victor W. Laurie (1965): "Structure of cyclopentadiene". ''The Journal of Chemical Physics'', volume 43, issue 8, pages 2765-2766. It is often abbreviated CpH beca .... Safety Its (intravenous, rabbit) ranges from 0.09 (male rabbit) to 0.11 ml/kg (female). It is also a neurotoxin. Referenc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
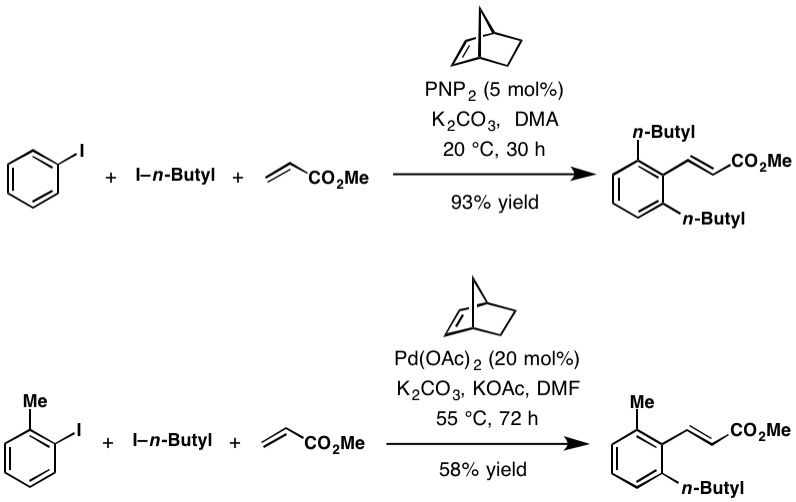
Catellani Reaction
The Catellani reaction was discovered by Marta Catellani ( Università degli Studi di Parma, Italy) and co-workers in 1997. The reaction uses aryl iodides to perform bi- or tri-functionalization, including C-H functionalization of the unsubstituted '' ortho'' position(s), followed a terminating cross-coupling reaction at the '' ipso'' position. This cross-coupling cascade reaction depends on the '' ortho'' -directing transient mediator, norbornene. Reaction mechanism The Catellani reaction is catalyzed by palladium and norbornene, although in most cases superstochiometric amounts of norbornene are used to allow the reaction to proceed at a reasonable rate. The generally accepted reaction mechanism, as outlined below, is intricate and believed to proceed via a series of Pd(0), Pd(II), and Pd(IV) intermediates, although an alternative bimetallic mechanism that avoids the formation of Pd(IV) has also been suggested. Initially, Pd(0) oxidatively adds into the C–X bond of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diels–Alder Reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a Conjugated system, conjugated diene and a substituted alkene, commonly termed the Diels–Alder reaction#The dienophile, dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted reaction, concerted mechanism. More specifically, it is classified as a thermally-allowed [4+2] cycloaddition with Woodward–Hoffmann rules, Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ring-opening Metathesis Polymerization
Ring-opening metathesis polymerization (ROMP) is a type of olefin metathesis chain-growth polymerization. The driving force of the reaction is relief of ring strain in cyclic olefins (e.g. norbornene or cyclopentene). A variety of heterogeneous and homogeneous catalysts have been developed. Most large-scale commercial processes rely on the former while some fine chemical syntheses rely on the homogeneous catalysts. Catalysts are based on transition metals such as W, Mo, Re, Ru, and Ti. Heterogeneous catalysis and applications : Ring-opening metathesis polymerization of cycloalkenes has been commercialized since the 1970s. Examples of polymers produced on an industrial level through ROMP catalysis are Vestenamer or trans-polyoctenamer, which is the metathetical polymer of cyclooctene. Norsorex or polynorbornene is another important ROMP product on the market. Telene and Metton are polydicyclopentadiene products produced in a side reaction of the polymerization of norbornene. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclic Olefin Copolymer
Cyclic olefin copolymer (COC) is an amorphous polymer made by several polymer manufacturers. COC is a relatively new class of polymers as compared to commodities such as polypropylene and polyethylene. This newer material is used in a wide variety of applications including packaging films, lenses, vials, displays, and medical devices. Various types In 2005 there were "several types of commercial cyclic olefin copolymers based on different types of cyclic monomers and polymerization methods. Cyclic olefin copolymers are produced by chain copolymerization of cyclic monomers such as 8,9,10-trinorborn-2-ene (norbornene) or 1,2,3,4,4a,5,8,8a-octahydro-1,4:5,8-dimethanonaphthalene (tetracyclododecene) with ethene (such as Polyplastics subsidiary TOPAS Advanced Polymers' TOPAS, Mitsui Chemical's APEL), or by ring-opening metathesis polymerization of various cyclic monomers followed by hydrogenation (Japan Synthetic Rubber's ARTON, Zeon Chemical's Zeonex and Zeonor)." These later material ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Norbornyl Cation
In organic chemistry, the term 2-norbornyl cation (or 2-bicyclo .2.1eptyl cation) describes one of the three carbocations formed from derivatives of norbornane. Though 1-norbornyl and 7-norbornyl cations have been studied, the most extensive studies and vigorous debates have been centered on the exact structure of the 2-norbornyl cation. The 2-norbornyl cation has been formed from a variety of norbornane derivatives and reagents. First reports of its formation and reactivity published by Saul Winstein sparked controversy over the nature of its bonding, as he invoked a three-center two-electron bond to explain the stereochemical outcome of the reaction. Herbert C. Brown challenged this assertion on the grounds that classical resonance structures could explain these observations without needing to adapt a new perspective of bonding. Both researchers' views had its supporters, and dozens of scientists contributed ingeniously designed experiments to provide evidence for one viewpoi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ring Strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are substantially smaller than the idealized value of approximately 109°. Because of their high strain, the heat of combustion for these small rings is elevated. Ring strain results from a combination of angle strain, conformational strain or Pitzer strain (torsional eclipsing interactions), and transannular strain, also known as van der Waals strain or Prelog strain. The simplest examples of angle strain are small cycloalkanes such as cyclopropane and cyclobutane. Ring strain energy can be attributed to the energy required for the distortion of bond and bond angles in order to close a ring. Ring strain energy is believed to be the cause of accelerated rates in altering ring reactions. Its interactions with traditional bond energies ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadiene
Cyclopentadiene is an organic compound with the formula C5H6.LeRoy H. Scharpen and Victor W. Laurie (1965): "Structure of cyclopentadiene". ''The Journal of Chemical Physics'', volume 43, issue 8, pages 2765-2766. It is often abbreviated CpH because the cyclopentadienyl anion is abbreviated Cp−. This colorless liquid has a strong and unpleasant odor. At room temperature, this cyclic diene dimerizes over the course of hours to give dicyclopentadiene via a Diels–Alder reaction. This dimer can be restored by heating to give the monomer. The compound is mainly used for the production of cyclopentene and its derivatives. It is popularly used as a precursor to the cyclopentadienyl anion (Cp−), an important ligand in cyclopentadienyl complexes in organometallic chemistry. Production and reactions Cyclopentadiene production is usually not distinguished from dicyclopentadiene since they interconvert. They are obtained from coal tar (about 10–20 g/tonne) and by stea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass Transition Temperature
The glass–liquid transition, or glass transition, is the gradual and reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle "glassy" state into a viscous or rubbery state as the temperature is increased. ISO 11357-2: Plastics – Differential scanning calorimetry – Part 2: Determination of glass transition temperature (1999). An amorphous solid that exhibits a glass transition is called a glass. The reverse transition, achieved by supercooling a viscous liquid into the glass state, is called vitrification. The glass-transition temperature ''T''g of a material characterizes the range of temperatures over which this glass transition occurs (as an experimental definition, typically marked as 100 s of relaxation time). It is always lower than the melting temperature, ''T''m, of the crystalline state of the material, if one exists. Hard plastics like polystyrene and poly(methyl methacrylate) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and hydrophobic, and their odors are usually weak or exemplified by the odors of gasoline and lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to the naturally occurring petroleum, natural gas and coal, and to their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene. Although butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles. The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene with structure H2C=C=CH−CH3. This allene has no industrial significance. History In 1863, the French chemist E. Caventou isolated butadiene from the pyrolysis of amyl alcohol. This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum. In 1910, the Russian chemist Sergei Lebedev polymerized butadiene and obtained a materi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Addition Polymerization
Chain-growth polymerization (American English, AE) or chain-growth polymerisation (British English, BE) is a polymerization technique where Unsaturated compound, unsaturated monomer molecules add onto the active site on a growing polymer chain one at a time. There are a limited number of these active sites at any moment during the polymerization which gives this method its key characteristics. Introduction In 1953, Paul Flory first classified polymerization as "step-growth polymerization" and "chain-growth polymerization". IUPAC recommends to further simplify "chain-growth polymerization" to "chain polymerization". It is a kind of polymerization where an active center (free radical or ion) is formed, and a plurality of monomers can be polymerized together in a short period of time to form a macromolecule having a large molecular weight. In addition to the regenerated active sites of each monomer unit, polymer growth will only occur at one (or possibly more) endpoint. Many comm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |