|
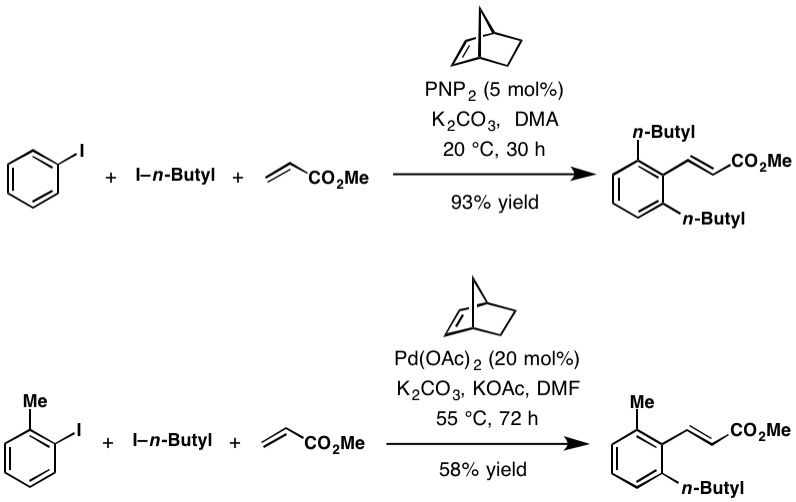
Catellani Reaction
The Catellani reaction was discovered by Marta Catellani (Università degli Studi di Parma, Italy) and co-workers in 1997. The reaction uses aryl iodides to perform bi- or tri-functionalization, including C-H functionalization of the unsubstituted '' ortho'' position(s), followed a terminating cross-coupling reaction at the '' ipso'' position. This cross-coupling cascade reaction depends on the '' ortho'' -directing transient mediator, norbornene. Reaction mechanism The Catellani reaction is catalyzed by palladium and norbornene, although in most cases superstochiometric amounts of norbornene are used to allow the reaction to proceed at a reasonable rate. The generally accepted reaction mechanism, as outlined below, is intricate and believed to proceed via a series of Pd(0), Pd(II), and Pd(IV) intermediates, although an alternative bimetallic mechanism that avoids the formation of Pd(IV) has also been suggested. Initially, Pd(0) oxidatively adds into the C–X bond of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Marta Catellani
Marta Catellani is an Italian chemist known for her discovery of the eponymous Catellani reaction in 1997. She was elected to the European Academy of Sciences in 2016. Catellani earned her Ph.D. in chemistry in 1971 from the University of Parma, where, as of 2019, she is a professor and chairs the Department of Organic Chemistry. Catellani completed her postdoctoral education at the University of Chicago. She has served as a visiting professor at Moscow State University (1992), Beijing Institute of Technology (2004), and University of Xi'an (2004). She was awarded a fellowship at the Japan Society for the Promotion of Science in 2012. Her research focuses on palladium Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ... as a catalyst for multistep organic reactions. The Catellani R ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidative Addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidative addition is often a step in catalytic cycles, in conjunction with its reverse reaction, reductive elimination. Role in transition metal chemistry For transition metals, oxidative reaction results in the decrease in the d''n'' to a configuration with fewer electrons, often 2e fewer. Oxidative addition is favored for metals that are (i) basic and/or (ii) easily oxidized. Metals with a relatively low oxidation state often satisfy one of these requirements, but even high oxidation state metals undergo oxidative addition, as illustrated by the oxidation of Pt(II) with chlorine: : tCl4sup>2− + Cl2 → tCl6sup>2− In classical organometallic chemistry, the formal oxidation state of the metal and the electron count of the complex both in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspidospermidine
Aspidospermidine is an alkaloid isolated from plants in the genus ''Aspidosperma''. It has been a popular target for total synthesis Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes ..., due in part to the fact that it provides a good showcase for synthetic strategies but also because the structure is similar to many other important bioactive molecules. References Alkaloids found in Apocynaceae Indolizidines {{alkaloid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhazinilam
Rhazinilam is an alkaloid first isolated in 1965 by Linde from the ''Melodinus australis'' plant. It was later isolated from the shrub ''Rhazya stricta'' as well as from other organisms. Biological activity Rhazinilam has activity similar to that of colchicine, taxol and vinblastine, acting as a spindle poison A spindle poison, also known as a spindle toxin, is a poison that disrupts cell division by affecting the protein threads that connect the centromere regions of chromosomes, known as spindles. Spindle poisons effectively cease the production of new .... Total synthesis Rhazinilam was first synthesized in 1973 by Smith and coworkers, and multiple subsequent times. Trauner synthesis :{{clear-left References Pyrroles Alkaloids Total synthesis Plant toxins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Total Synthesis
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes it from semisynthesis. Syntheses may sometimes conclude at a precursor with further known synthetic pathways to a target molecule, in which case it is known as a formal synthesis. Total synthesis target molecules can be natural products, medicinally-important active ingredients, known intermediates, or molecules of theoretical interest. Total synthesis targets can also be organometallic or inorganic, though these are rarely encountered. Total synthesis projects often require a wide diversity of reactions and reagents, and subsequently requires broad chemical knowledge and training to be successful. Often, the aim is to discover a new route of synthesis for a target molecule for which there already exist known routes. Sometimes, however, no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkynylation
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne () is added to a carbonyl group () to form an Alpha and beta carbon, α-alkynyl alcohol (chemistry), alcohol (). When the acetylide is formed from acetylene (), the reaction gives an α-ethynyl alcohol. This process is often referred to as ethynylation. Such processes often involve metal acetylide intermediates. Scope The principal reaction of interest involves the addition of the acetylene () to a ketone () or aldehyde (): :RR'C=O + HC#CR'' -> RR'C(OH)C#CR'' The reaction proceeds with retention of the triple bond. For aldehydes and unsymmetrical ketones, the product is chiral, hence there is interest in asymmetric variants. These reactions invariably involve metal-acetylide intermediates. This reaction was discovered by chemist John Ulric Nef (chemist), John Ulric Nef in 1899 while experimenting with reactions of elemental sodium, phenylacetylene, and acetophenone. For this reason, the re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isopropyl Alcohol
Isopropyl alcohol (IUPAC name propan-2-ol and also called isopropanol or 2-propanol) is a colorless, flammable organic compound with a pungent alcoholic odor. As an isopropyl group linked to a hydroxyl group (chemical formula ) it is the simplest example of a secondary alcohol, where the alcohol carbon atom is attached to two other carbon atoms. It is a structural isomer of propan-1-ol and ethyl methyl ether. It is used in the manufacture of a wide variety of industrial and household chemicals and is a common ingredient in products such as antiseptics, disinfectants, hand sanitizer and detergents. Well over one million tonnes is produced worldwide annually. Properties Isopropyl alcohol is miscible in water, ethanol, and chloroform as, like these compounds, isopropyl is a polar molecule. It dissolves ethyl cellulose, polyvinyl butyral, many oils, alkaloids, and natural resins. Unlike ethanol or methanol, isopropyl alcohol is not miscible with salt solutions and can be separ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bis(pinacolato)diboron
Bis(pinacolato)diboron is a covalent compound containing two boron atoms and two pinacolato ligands. It has the formula CH3)4C2O2Bsub>2; the pinacol groups are sometimes abbreviated as "pin", so the structure is sometimes represented as B2pin2. It is a colourless solid that is soluble in organic solvents. It is a commercially available reagent for making pinacol boronic esters for organic synthesis. Unlike some other diboron compounds, B2pin2 is not moisture-sensitive and can be handled in air. Preparation and structure This compound may be prepared by treating tetrakis(dimethylamino)diboron with pinacol Pinacol is a white solid organic compound. It is a diol that has hydroxyl groups (-OH) on vicinal carbon atoms. Preparation It may be produced by the pinacol coupling reaction from acetone: Reactions As a vicinal-diol, it can rearrange t ... in acidic conditions. The B-B bond length is 1.711(6) Å. Dehydrogenation of pinacolborane provides an alternative route: :2(CH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borylation
Metal-catalyzed C–H borylation reactions are transition metal catalyzed organic reactions that produce an organoboron compound through functionalization of aliphatic and aromatic C–H bonds and are therefore useful reactions for carbon–hydrogen bond activation. Metal-catalyzed C–H borylation reactions utilize transition metals to directly convert a C–H bond into a C–B bond. This route can be advantageous compared to traditional borylation reactions by making use of cheap and abundant hydrocarbon starting material, limiting prefunctionalized organic compounds, reducing toxic byproducts, and streamlining the synthesis of biologically important molecules. Boronic acids, and boronic esters are common boryl groups incorporated into organic molecules through borylation reactions. Boronic acids are trivalent boron-containing organic compounds that possess one alkyl substituent and two hydroxyl groups. Similarly, boronic esters possess one alkyl substituent and two ester groups. Bo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boronic Ester
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, members of this class thus belong to the larger class of organoboranes. Boronic acids act as Lewis acids. Their unique feature is that they are capable of forming reversible covalent complexes with sugars, amino acids, hydroxamic acids, etc. (molecules with vicinal, (1,2) or occasionally (1,3) substituted Lewis base donors (alcohol, amine, carboxylate)). The p''K''a of a boronic acid is ~9, but they can form tetrahedral boronate complexes with p''K''a ~7. They are occasionally used in the area of molecular recognition to bind to saccharides for fluorescent detection or selective transport of saccharides across membranes. Boronic acids are used extensively in organic chemistry as chemical building blocks and intermediates predominantly in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond. Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, and Biological Chemistry'. 1232 pages. Two general types of monoalkenes are distinguished: terminal and internal. Also called α-olefins, terminal alkenes are more useful. However, the International Union of Pure and Applied Chemistry (IUPAC) recommends using the name "alkene" only for acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with '' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |