Catellani Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Catellani reaction was discovered by 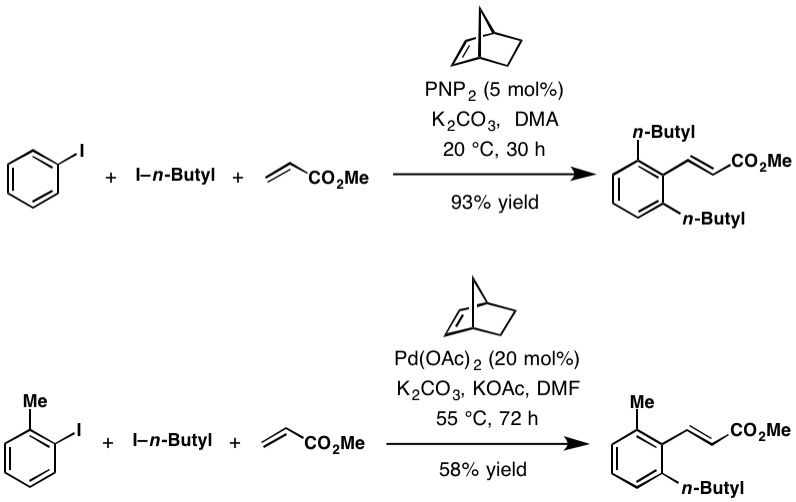
Total Synthesis of (+)-Linoxepin by Utilizing the Catellani Reaction
{{Organic reactions Chemical reactions
Marta Catellani
Marta Catellani is an Italian chemist known for her discovery of the eponymous Catellani reaction in 1997. She was elected to the European Academy of Sciences in 2016. Catellani earned her Ph.D. in chemistry in 1971 from the University of Parma, w ...
(Università degli Studi di Parma
The University of Parma ( it, Università degli Studi di Parma, UNIPR) is a public university in Parma, Emilia-Romagna, Italy. It is organised in nine departments. As of 2016 the University of Parma has about 26,000 students.
History
During the ...
, Italy) and co-workers in 1997. The reaction uses aryl iodides to perform bi- or tri-functionalization, including C-H functionalization of the unsubstituted '' ortho'' position(s), followed a terminating cross-coupling reaction at the '' ipso'' position
Position often refers to:
* Position (geometry), the spatial location (rather than orientation) of an entity
* Position, a job or occupation
Position may also refer to:
Games and recreation
* Position (poker), location relative to the dealer
* ...
. This cross-coupling
In organic chemistry, a cross-coupling reaction is a reaction where two fragments are joined together with the aid of a metal catalyst. In one important reaction type, a main group organometallic compound of the type R-M (R = organic fragment, M = ...
cascade reaction
A cascade reaction, also known as a domino reaction or tandem reaction, is a chemical process that comprises at least two consecutive reactions such that each subsequent reaction occurs only in virtue of the chemical functionality formed in the p ...
depends on the '' ortho'' -directing transient mediator, norbornene.

Reaction mechanism
The Catellani reaction is catalyzed bypalladium
Palladium is a chemical element with the symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1803 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas, which was itself na ...
and norbornene, although in most cases superstochiometric amounts of norbornene are used to allow the reaction to proceed at a reasonable rate. The generally accepted reaction mechanism, as outlined below, is intricate and believed to proceed via a series of Pd(0), Pd(II), and Pd(IV) intermediates, although an alternative bimetallic mechanism that avoids the formation of Pd(IV) has also been suggested.
Initially, Pd(0) oxidatively adds into the C–X bond of the aryl halide. Subsequently, the arylpalladium(II) species undergoes carbopalladation with the norbornene. The structure of the norbornylpalladium intermediate does not allow for β-hydride elimination at either of the β-positions due to Bredt's Rule Bredt's rule is an empirical observation in organic chemistry that states that a double bond cannot be placed at the bridgehead of a bridged ring system, unless the rings are large enough. The rule is named after Julius Bredt, who first discussed i ...
for the bridgehead β-hydrogen and the ''trans-''configuration between palladium and other β-hydrogen. Thereafter, the Pd(II) species undergoes electrophilic cyclopalladation at the ''ortho'' position of the aryl group. Subsequently, the palladacyclic intermediate undergoes a second oxidation addition with the alkyl halide coupling partner to form a Pd(IV) intermediate, which undergoes reductive elimination to forge the first C–C bond of the product. After β-carbon elimination of norbornene, the resultant Pd(II) species then undergoes a second C–C bond forming step via a Heck reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a s ...
or cross coupling with an organoboron reagent to afford the final organic product and close the catalytic cycle.
Steps of the Catellani reaction:
# Oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidat ...
# Carbopalladation of norbornene
# Palladacycle formation
# Oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxidat ...
to palladacycle
# Reductive elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and is ...
from palladacycle
# Norbornene extrusion
# Termination via Heck reaction
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence of a base and a palladium catalyst (or palladium nanomaterial-based catalyst) to form a s ...
, Suzuki reaction
The Suzuki reaction is an organic reaction, classified as a cross-coupling reaction, where the coupling partners are a boronic acid and an organohalide and the catalyst is a palladium(0) complex. It was first published in 1979 by Akira Suzuki, ...
, etc.
''Ortho'' and ''ipso'' cross-coupling partners
The Catellani reaction facilitates a variety of C—C and C—N bond-forming reactions at the ''ortho'' position. These include alkylation from alkyl halides, arylation from aryl bromides, amination from benzyloxyamines, acylation from anhydrides. Likewise in the case of terminating ''ipso'' coupling partners with Heck-type termination with olefins,Suzuki
is a Japan, Japanese multinational corporation headquartered in Minami-ku, Hamamatsu, Japan. Suzuki manufactures automobiles, motorcycles, All-terrain vehicle, all-terrain vehicles (ATVs), outboard motor, outboard marine engines, wheelchairs ...
-type reaction with boronic ester
A boronic acid is an organic compound related to boric acid () in which one of the three hydroxyl groups () is replaced by an alkyl or aryl group (represented by R in the general formula ). As a compound containing a carbon–boron bond, memb ...
s, borylation with bis(pinacolato)diboron
Bis(pinacolato)diboron is a covalent compound containing two boron atoms and two pinacolato ligands. It has the formula CH3)4C2O2Bsub>2; the pinacol groups are sometimes abbreviated as "pin", so the structure is sometimes represented as B2pin2. It ...
, protonation with ''i''-PrOH, decarboxylative alkynylation
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne () is added to a carbonyl group () to form an Alpha and beta carbon, α-alkynyl alcohol (chemistry), alcohol ().
When the acetylide is formed from acetylene () ...
with alkynyl carboxylic acids.
Uses
With tethered cross-coupling partners, Lautens, Malacria, and Catellani used this reaction to construct a variety of fused ring systems since 2000. The Catellani reaction has been used as a key step for thetotal synthesis
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes ...
(+)- linoxepin, rhazinal, aspidospermidine, and (±)- goniomitine.
References
External links
Total Synthesis of (+)-Linoxepin by Utilizing the Catellani Reaction
{{Organic reactions Chemical reactions