|
Maltase
Maltase (, ''alpha-glucosidase'', ''glucoinvertase'', ''glucosidosucrase'', ''maltase-glucoamylase'', ''alpha-glucopyranosidase'', ''glucosidoinvertase'', ''alpha-D-glucosidase'', ''alpha-glucoside hydrolase'', ''alpha-1,4-glucosidase'', ''alpha-D-glucoside glucohydrolase'') is one type of alpha-glucosidase enzymes located in the brush border of the small intestine. This enzyme catalyzes the hydrolysis of disaccharide maltose into two simple sugars of glucose. Maltase is found in plants, bacteria, yeast, humans, and other vertebrates. It is thought to be synthesized by cells of the mucous membrane lining the intestinal wall. Digestion of starch requires six intestinal enzymes. Two of these enzymes are luminal endo-glucosidases named alpha-amylases. The other four enzymes have been identified as different maltases, exo-glucosidases bound to the luminal surface of enterocytes. Two of these maltase activities were associated with sucrase-isomaltase (maltase Ib, maltase Ia). The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sucrase-isomaltase
Oligo-1,6-glucosidase (EC 3.2.1.10, sucrase-isomaltase, SI; systematic name oligosaccharide 6-α-glucohydrolase) is a glucosidase enzyme located on the brush border of the small intestine, which catalyses the following reaction: :Hydrolysis of (1→6)-α-D-glucosidic linkages in some oligosaccharides produced from starch and glycogen by (α-amylase), and in isomaltose It is a dual-function enzyme with two GH31 domains, one serving as the isomaltase, the other as a sucrose alpha-glucosidase. It has preferential expression in the apical membranes of enterocytes. The enzyme’s purpose is to digest dietary carbohydrates such as starch, sucrose and isomaltose. By further processing the broken-down products, energy in the form of ATP can be generated.Berg, J. M. et al. ''Biochemistry'', 7th Ed. W.H. Freeman and Company: New York, 2012. Structure Sucrase-isomaltase consists of two enzymatic subunits: sucrase and isomaltase. The subunits originate from a polypeptide precurs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sucrase-isomaltase
Oligo-1,6-glucosidase (EC 3.2.1.10, sucrase-isomaltase, SI; systematic name oligosaccharide 6-α-glucohydrolase) is a glucosidase enzyme located on the brush border of the small intestine, which catalyses the following reaction: :Hydrolysis of (1→6)-α-D-glucosidic linkages in some oligosaccharides produced from starch and glycogen by (α-amylase), and in isomaltose It is a dual-function enzyme with two GH31 domains, one serving as the isomaltase, the other as a sucrose alpha-glucosidase. It has preferential expression in the apical membranes of enterocytes. The enzyme’s purpose is to digest dietary carbohydrates such as starch, sucrose and isomaltose. By further processing the broken-down products, energy in the form of ATP can be generated.Berg, J. M. et al. ''Biochemistry'', 7th Ed. W.H. Freeman and Company: New York, 2012. Structure Sucrase-isomaltase consists of two enzymatic subunits: sucrase and isomaltase. The subunits originate from a polypeptide precurs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltase Reaction
Maltase (, ''alpha-glucosidase'', ''glucoinvertase'', ''glucosidosucrase'', ''maltase-glucoamylase'', ''alpha-glucopyranosidase'', ''glucosidoinvertase'', ''alpha-D-glucosidase'', ''alpha-glucoside hydrolase'', ''alpha-1,4-glucosidase'', ''alpha-D-glucoside glucohydrolase'') is one type of alpha-glucosidase enzymes located in the brush border of the small intestine. This enzyme catalyzes the hydrolysis of disaccharide maltose into two simple sugars of glucose. Maltase is found in plants, bacteria, yeast, humans, and other vertebrates. It is thought to be synthesized by cells of the mucous membrane lining the intestinal wall. Digestion of starch requires six intestinal enzymes. Two of these enzymes are luminal endo-glucosidases named alpha-amylases. The other four enzymes have been identified as different maltases, exo-glucosidases bound to the luminal surface of enterocytes. Two of these maltase activities were associated with sucrase-isomaltase (maltase Ib, maltase Ia). The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltase-glucoamylase
Maltase-glucoamylase, intestinal is an enzyme that in humans is encoded by the ''MGAM'' gene. Maltase-glucoamylase is an alpha-glucosidase digestive enzyme. It consists of two subunits with differing substrate specificity. Recombinant enzyme studies have shown that its N-terminal catalytic domain has highest activity against maltose, while the C-terminal domain has a broader substrate specificity and activity against glucose oligomers. In the small intestine, this enzyme works in synergy with sucrase-isomaltase and alpha-amylase to digest the full range of dietary starches. Gene The MGAM gene –– which is located on chromosome 7q34 –– codes for the protein Maltase-Glucoamylase. An alternative name for Maltase-Glucoamylase is glucan 1,4-alpha-glycosidase. Tissue distribution Maltase-glucoamylase is a membrane-bound enzyme located in the intestinal walls. This lining of the intestine forms brush border in which food has to pass in order for the intestines to absor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltase-glucoamylase
Maltase-glucoamylase, intestinal is an enzyme that in humans is encoded by the ''MGAM'' gene. Maltase-glucoamylase is an alpha-glucosidase digestive enzyme. It consists of two subunits with differing substrate specificity. Recombinant enzyme studies have shown that its N-terminal catalytic domain has highest activity against maltose, while the C-terminal domain has a broader substrate specificity and activity against glucose oligomers. In the small intestine, this enzyme works in synergy with sucrase-isomaltase and alpha-amylase to digest the full range of dietary starches. Gene The MGAM gene –– which is located on chromosome 7q34 –– codes for the protein Maltase-Glucoamylase. An alternative name for Maltase-Glucoamylase is glucan 1,4-alpha-glycosidase. Tissue distribution Maltase-glucoamylase is a membrane-bound enzyme located in the intestinal walls. This lining of the intestine forms brush border in which food has to pass in order for the intestines to absor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
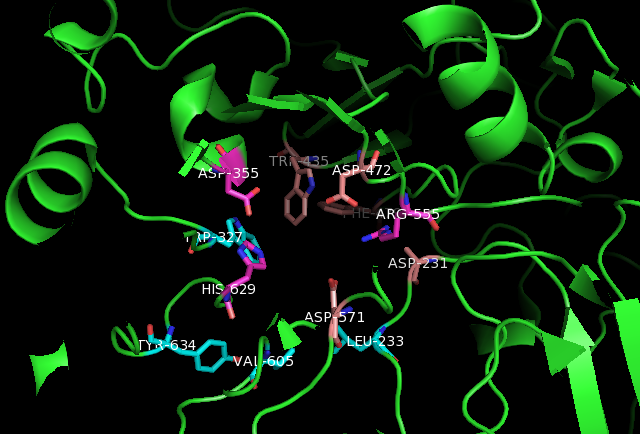
Maltase-Glucoamylase Ligand Interactions
Maltase-glucoamylase, intestinal is an enzyme that in humans is encoded by the ''MGAM'' gene. Maltase-glucoamylase is an alpha-glucosidase digestive enzyme. It consists of two subunits with differing substrate specificity. Recombinant enzyme studies have shown that its N-terminal catalytic domain has highest activity against maltose, while the C-terminal domain has a broader substrate specificity and activity against glucose oligomers. In the small intestine, this enzyme works in synergy with sucrase-isomaltase and alpha-amylase to digest the full range of dietary starches. Gene The MGAM gene –– which is located on chromosome 7q34 –– codes for the protein Maltase-Glucoamylase. An alternative name for Maltase-Glucoamylase is glucan 1,4-alpha-glycosidase. Tissue distribution Maltase-glucoamylase is a membrane-bound enzyme located in the intestinal walls. This lining of the intestine forms brush border in which food has to pass in order for the intestines to absor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alpha-glucosidase
α-Glucosidase (EC 3.2.1.20, maltase, glucoinvertase, glucosidosucrase, maltase-glucoamylase, α-glucopyranosidase, glucosidoinvertase, α-D-glucosidase, α-glucoside hydrolase, α-1,4-glucosidase, α-D-glucoside glucohydrolase; systematic name α-D-glucoside glucohydrolase) is a glucosidase located in the brush border of the small intestine that acts upon α(1→4) bonds: : Hydrolysis of terminal, non-reducing (1→4)-linked α-D-glucose residues with release of D-glucose This is in contrast to β-glucosidase. α-Glucosidase breaks down starch and disaccharides to glucose. Other glucosidases include: * Cellulase * Beta-glucosidase * Debranching enzyme Mechanism α-Glucosidase hydrolyzes terminal non-reducing (1→4)-linked α-glucose residues to release a single α-glucose molecule. α-Glucosidase is a carbohydrate-hydrolase that releases α-glucose as opposed to β-glucose. β-Glucose residues can be released by glucoamylase, a functionally similar enzyme. The substr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltose Structure
} Maltose ( or ), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the amylose homologous series, the key structural motif of starch. When beta-amylase breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in germinating seeds, which is why it was named after malt. Unlike sucrose, it is a reducing sugar. History Maltose was discovered by Augustin-Pierre Dubrunfaut, although this discovery was not widely accepted until it was confirmed in 1872 by Irish chemist and brewer Cornelius O'Sullivan. Its name comes from malt, combined with the suffix '-ose' which is used in names of sugars. Structure and nomenclature Carbohydrates are generally divided into monosaccharides, oligosaccharides, and polysaccharides depending ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltose
} Maltose ( or ), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the amylose homologous series, the key structural motif of starch. When beta-amylase breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in germinating seeds, which is why it was named after malt. Unlike sucrose, it is a reducing sugar. History Maltose was discovered by Augustin-Pierre Dubrunfaut, although this discovery was not widely accepted until it was confirmed in 1872 by Irish chemist and brewer Cornelius O'Sullivan. Its name comes from malt, combined with the suffix ' -ose' which is used in names of sugars. Structure and nomenclature Carbohydrates are generally divided into monosaccharides, oligosaccharides, and polysaccharides depending on the nu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glucose
Glucose is a simple sugar with the molecular formula . Glucose is overall the most abundant monosaccharide, a subcategory of carbohydrates. Glucose is mainly made by plants and most algae during photosynthesis from water and carbon dioxide, using energy from sunlight, where it is used to make cellulose in cell walls, the most abundant carbohydrate in the world. In energy metabolism, glucose is the most important source of energy in all organisms. Glucose for metabolism is stored as a polymer, in plants mainly as starch and amylopectin, and in animals as glycogen. Glucose circulates in the blood of animals as blood sugar. The naturally occurring form of glucose is -glucose, while -glucose is produced synthetically in comparatively small amounts and is less biologically active. Glucose is a monosaccharide containing six carbon atoms and an aldehyde group, and is therefore an aldohexose. The glucose molecule can exist in an open-chain (acyclic) as well as ring (cyclic) form. Gluco ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid Alpha-glucosidase
Acid alpha-glucosidase, also called α-1,4-glucosidase and acid maltase, is an enzyme () that helps to break down glycogen in the lysosome. It is functionally similar to glycogen debranching enzyme, but is on a different chromosome, processed differently by the cell and is located in the lysosome rather than the cytosol. In humans, it is encoded by the ''GAA'' gene. Errors in this gene cause glycogen storage disease type II (Pompe disease). Function This gene encodes lysosomal alpha-glucosidase, which is essential for the degradation of glycogen to glucose in lysosome A lysosome () is a membrane-bound organelle found in many animal cells. They are spherical vesicles that contain hydrolytic enzymes that can break down many kinds of biomolecules. A lysosome has a specific composition, of both its membrane prot ...s. Different forms of acid alpha-glucosidase are obtained by proteolytic processing. Defects in this gene are the cause of glycogen storage disease II, also known a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polysaccharide
Polysaccharides (), or polycarbohydrates, are the most abundant carbohydrates found in food. They are long chain polymeric carbohydrates composed of monosaccharide units bound together by glycosidic linkages. This carbohydrate can react with water (hydrolysis) using amylase enzymes as catalyst, which produces constituent sugars (monosaccharides, or oligosaccharides). They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch, glycogen and galactogen and structural polysaccharides such as cellulose and chitin. Polysaccharides are often quite heterogeneous, containing slight modifications of the repeating unit. Depending on the structure, these macromolecules can have distinct properties from their monosaccharide building blocks. They may be amorphous or even insoluble in water. When all the monosaccharides in a polysaccharide are the same type, the polysaccharide is called a homopolysaccharide or homoglycan, but when more t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |