|
Maltose
} Maltose ( or ), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two-unit member of the amylose homologous series, the key structural motif of starch. When beta-amylase breaks down starch, it removes two glucose units at a time, producing maltose. An example of this reaction is found in germinating seeds, which is why it was named after malt. Unlike sucrose, it is a reducing sugar. History Maltose was discovered by Augustin-Pierre Dubrunfaut, although this discovery was not widely accepted until it was confirmed in 1872 by Irish chemist and brewer Cornelius O'Sullivan. Its name comes from malt, combined with the suffix ' -ose' which is used in names of sugars. Structure and nomenclature Carbohydrates are generally divided into monosaccharides, oligosaccharides, and polysaccharides depending on the nu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Starch
Starch or amylum is a polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants for energy storage. Worldwide, it is the most common carbohydrate in human diets, and is contained in large amounts in staple foods such as wheat, potatoes, maize (corn), rice, and cassava (manioc). Pure starch is a white, tasteless and odorless powder that is insoluble in cold water or alcohol. It consists of two types of molecules: the linear and helical amylose and the branched amylopectin. Depending on the plant, starch generally contains 20 to 25% amylose and 75 to 80% amylopectin by weight. Glycogen, the energy reserve of animals, is a more highly branched version of amylopectin. In industry, starch is often converted into sugars, for example by malting. These sugars may be fermented to produce ethanol in the manufacture of beer, whisky and biofuel. In addition, sugars produced from processed starch are used ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomaltose
Isomaltose is a disaccharide similar to maltose, but with a α-(1-6)-linkage instead of the α-(1-4)-linkage. Both of the sugars are dimers of glucose, which is a pyranose sugar. Isomaltose is a reducing sugar. Isomaltose is produced when high maltose syrup is treated with the enzyme transglucosidase (TG) and is one of the major components in the mixture isomaltooligosaccharide. It is a product of the caramelization of glucose. See also *Isomaltase *Maltose } Maltose ( or ), also known as maltobiose or malt sugar, is a disaccharide formed from two units of glucose joined with an α(1→4) bond. In the isomer isomaltose, the two glucose molecules are joined with an α(1→6) bond. Maltose is the two- ... References External links * Disaccharides {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maltase
Maltase (, ''alpha-glucosidase'', ''glucoinvertase'', ''glucosidosucrase'', ''maltase-glucoamylase'', ''alpha-glucopyranosidase'', ''glucosidoinvertase'', ''alpha-D-glucosidase'', ''alpha-glucoside hydrolase'', ''alpha-1,4-glucosidase'', ''alpha-D-glucoside glucohydrolase'') is one type of alpha-glucosidase enzymes located in the brush border of the small intestine. This enzyme catalyzes the hydrolysis of disaccharide maltose into two simple sugars of glucose. Maltase is found in plants, bacteria, yeast, humans, and other vertebrates. It is thought to be synthesized by cells of the mucous membrane lining the intestinal wall. Digestion of starch requires six intestinal enzymes. Two of these enzymes are luminal endo-glucosidases named alpha-amylases. The other four enzymes have been identified as different maltases, exo-glucosidases bound to the luminal surface of enterocytes. Two of these maltase activities were associated with sucrase-isomaltase (maltase Ib, maltase Ia). The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amylase Reaction
An amylase () is an enzyme that catalyses the hydrolysis of starch (Latin ') into sugars. Amylase is present in the saliva of humans and some other mammals, where it begins the chemical process of digestion. Foods that contain large amounts of starch but little sugar, such as rice and potatoes, may acquire a slightly sweet taste as they are chewed because amylase degrades some of their starch into sugar. The pancreas and salivary gland make amylase (alpha amylase) to hydrolyse dietary starch into disaccharides and trisaccharides which are converted by other enzymes to glucose to supply the body with energy. Plants and some bacteria also produce amylase. Specific amylase proteins are designated by different Greek letters. All amylases are glycoside hydrolases and act on α-1,4-glycosidic bonds. Classification α-Amylase The α-amylases () ( CAS 9014-71-5) (alternative names: 1,4-α-D-glucan glucanohydrolase; glycogenase) are calcium metalloenzymes. By acting at random loca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disaccharide
A disaccharide (also called a double sugar or ''biose'') is the sugar formed when two monosaccharides are joined by glycosidic linkage. Like monosaccharides, disaccharides are simple sugars soluble in water. Three common examples are sucrose, lactose, and maltose. Disaccharides are one of the four chemical groupings of carbohydrates (monosaccharides, disaccharides, oligosaccharides, and polysaccharides). The most common types of disaccharides—sucrose, lactose, and maltose—have 12 carbon atoms, with the general formula C12H22O11. The differences in these disaccharides are due to atomic arrangements within the molecule. The joining of monosaccharides into a double sugar happens by a condensation reaction, which involves the elimination of a water molecule from the functional groups only. Breaking apart a double sugar into its two monosaccharides is accomplished by hydrolysis with the help of a type of enzyme called a disaccharidase. As building the larger sugar ejects a water ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Beta-amylase
β-Amylase (EC 3.2.1.2 , saccharogen amylase, glycogenase) is an enzyme with the systematic name ''4-α-D-glucan maltohydrolase''. It catalyses the following reaction: : Hydrolysis of (1→4)-α-D-glucosidic linkages in polysaccharides so as to remove successive maltose units from the non-reducing ends of the chains This enzyme acts on starch, glycogen and related polysaccharides and oligosaccharides producing beta-maltose by an inversion. Beta-amylase is found in bacteria, fungi, and plants; bacteria and cereal sources are the most heat stable. Working from the non-reducing end, β-amylase catalyzes the hydrolysis of the second α-1,4 glycosidic bond, cleaving off two glucose units (maltose) at a time. During the ripening of fruit, β-amylase breaks starch into maltose, resulting in the sweet flavor of ripe fruit. β-amylase is present in an inactive form prior to seed germination. Many microbes also produce amylase to degrade extracellular starches. Animal tissues do not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reducing Sugar
A reducing sugar is any sugar that is capable of acting as a reducing agent. In an alkaline solution, a reducing sugar forms some aldehyde or ketone, which allows it to act as a reducing agent, for example in Benedict's reagent. In such a reaction, the sugar becomes a carboxylic acid. All monosaccharides are reducing sugars, along with some disaccharides, some oligosaccharides, and some polysaccharides. The monosaccharides can be divided into two groups: the aldoses, which have an aldehyde group, and the ketoses, which have a ketone group. Ketoses must first tautomerize to aldoses before they can act as reducing sugars. The common dietary monosaccharides galactose, glucose and fructose are all reducing sugars. Disaccharides are formed from two monosaccharides and can be classified as either reducing or nonreducing. Nonreducing disaccharides like sucrose and trehalose have glycosidic bonds between their anomeric carbons and thus cannot convert to an open-chain form with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malt
Malt is germinated cereal grain that has been dried in a process known as " malting". The grain is made to germinate by soaking in water and is then halted from germinating further by drying with hot air. Malted grain is used to make beer, whisky, malted milk, malt vinegar, confections such as Maltesers and Whoppers, flavored drinks such as Horlicks, Ovaltine, and Milo, and some baked goods, such as malt loaf, bagels, and Rich Tea biscuits. Malted grain that has been ground into a coarse meal is known as "sweet meal". Malting grain develops the enzymes (α-amylase, β-amylase) required for modifying the grains' starches into various types of sugar, including monosaccharide glucose, disaccharide maltose, trisaccharide maltotriose, and higher sugars called maltodextrines. It also develops other enzymes, such as proteases, that break down the proteins in the grain into forms that can be used by yeast. The point at which the malting process is stopped affects the starch-to-enz ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactose
Lactose is a disaccharide sugar synthesized by galactose and glucose subunits and has the molecular formula C12H22O11. Lactose makes up around 2–8% of milk (by mass). The name comes from ' (gen. '), the Latin word for milk, plus the suffix '' -ose'' used to name sugars. The compound is a white, water-soluble, non-hygroscopic solid with a mildly sweet taste. It is used in the food industry. Structure and reactions Lactose is a disaccharide derived from the condensation of galactose and glucose, which form a β-1→4 glycosidic linkage. Its systematic name is β-D-galactopyranosyl-(1→4)-D-glucose. The glucose can be in either the α-pyranose form or the β-pyranose form, whereas the galactose can only have the β-pyranose form: hence α-lactose and β-lactose refer to the anomeric form of the glucopyranose ring alone. Detection reactions for lactose are the Woehlk- and Fearon's test. Both can be easily used in school experiments to visualise the different lactose content o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
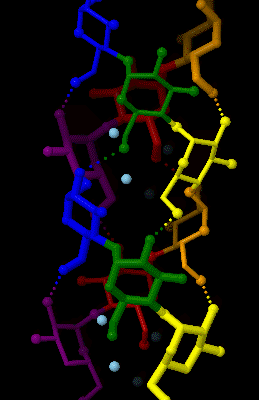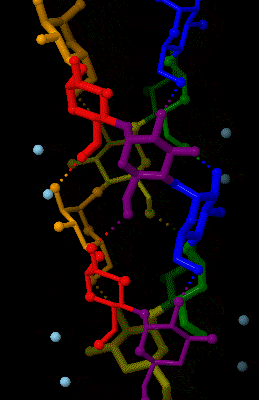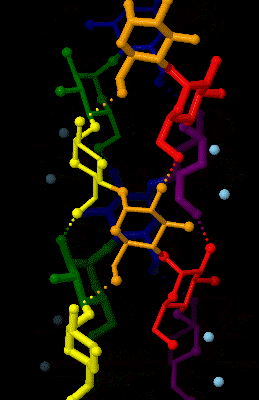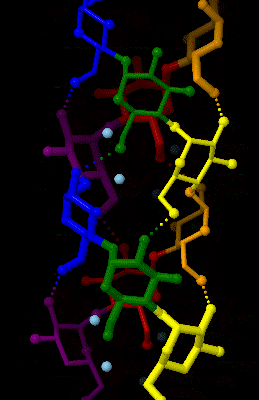
Amylose
Amylose is a polysaccharide made of α-D-glucose units, bonded to each other through α(1→4) glycosidic bonds. It is one of the two components of starch, making up approximately 20–30%. Because of its tightly packed helical structure, amylose is more resistant to digestion than other starch molecules and is therefore an important form of resistant starch. Structure Amylose is made up of α(1→4) bound glucose molecules. The carbon atoms on glucose are numbered, starting at the aldehyde (C=O) carbon, so, in amylose, the 1-carbon on one glucose molecule is linked to the 4-carbon on the next glucose molecule (α(1→4) bonds). The structural formula of amylose is pictured at right. The number of repeated glucose subunits (n) is usually in the range of 300 to 3000, but can be many thousands. There are three main forms of amylose chains can take. It can exist in a disordered amorphous conformation or two different helical forms. It can bind with itself in a double helix (A or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Augustin-Pierre Dubrunfaut
Augustin-Pierre Dubrunfaut (; 1 September 1797, Lille – 7 October 1881) was a French chemist. Mutarotation was discovered by Dubrunfaut in 1844, when he noticed that the specific rotation of aqueous sugar solution changes with time. In the same paper, he also identified that the inversion of sucrose in the presence of brewer's yeast (''Saccharomyces cerevisiae'') was not a consequence of fermentation. The organic fructose molecule was subsequently discovered by Dubrunfaut in 1847. He also discovered maltose, although this discovery was not widely accepted until it was confirmed in 1872 by Cornelius O'Sullivan Cornelius O'Sullivan, FRS (20 December 1841 – 8 January 1907) was an Irish brewer's chemist. He was born the son of merchant James O'Sullivan of Bandon, County Cork and was educated locally, before winning a scholarship to study chemistry at th .... Works * ''Art de fabriquer le sucre de betteraves, contenant 1. la description des meilleures méthodes usitées pour la c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homologous Series
In organic chemistry, a homologous series is a sequence of compounds with the same functional group and similar chemical properties in which the members of the series can be branched or unbranched, or differ by molecular formula of and molecular mass of 14u. This can be the length of a carbon chain, for example in the straight-chained alkanes (paraffins), or it could be the number of monomers in a homopolymer such as amylose. Compounds within a homologous series typically have a fixed set of functional groups that gives them similar chemical and physical properties. (For example, the series of primary straight-chained alcohols has a hydroxyl at the end of the carbon chain.) These properties typically change gradually along the series, and the changes can often be explained by mere differences in molecular size and mass. The name "homologous series" is also often used for any collection of compounds that have similar structures or include the same functional group, such as the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


_from_methane_to_nonadecane.png)