|
Magnesium Sulfate
Magnesium sulfate or magnesium sulphate (in English-speaking countries other than the US) is a chemical compound, a salt with the formula , consisting of magnesium cations (20.19% by mass) and sulfate anions . It is a white crystalline solid, soluble in water but not in ethanol. Magnesium sulfate is usually encountered in the form of a hydrate , for various values of ''n'' between 1 and 11. The most common is the heptahydrate , known as Epsom salt, which is a household chemical with many traditional uses, including bath salts. The main use of magnesium sulfate is in agriculture, to correct soils deficient in magnesium (an essential plant nutrient because of the role of magnesium in chlorophyll and photosynthesis). The monohydrate is favored for this use; by the mid 1970s, its production was 2.3 million tons per year. The anhydrous form and several hydrates occur in nature as minerals, and the salt is a significant component of the water from some springs. Hydrates Magnesi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epsomite
Epsomite, Epsom salt, or magnesium sulfate heptahydrate, is a hydrous magnesium sulfate mineral with formula MgSO4·7H2O. Epsomite crystallizes in the orthorhombic system as rarely found acicular or fibrous crystals, the normal form is as massive encrustations. It is colorless to white with tints of yellow, green and pink. The Mohs hardness is 2 to 2.5 and it has a low specific gravity of 1.67. It is readily soluble in water. It absorbs water from the air and converts to hexahydrate with the loss of one water molecule and a switch to monoclinic structure. Etymology It was first systematically described in 1806 for an occurrence near Epsom, Surrey, England, after which it was named. Discovery and occurrence Epsomite forms as encrustations or efflorescences on limestone cavern walls and mine timbers and walls, rarely as volcanic fumarole deposits, and as rare beds in evaporite layers such as those found in certain bodies of salt water. It occurs in association with melanterite, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
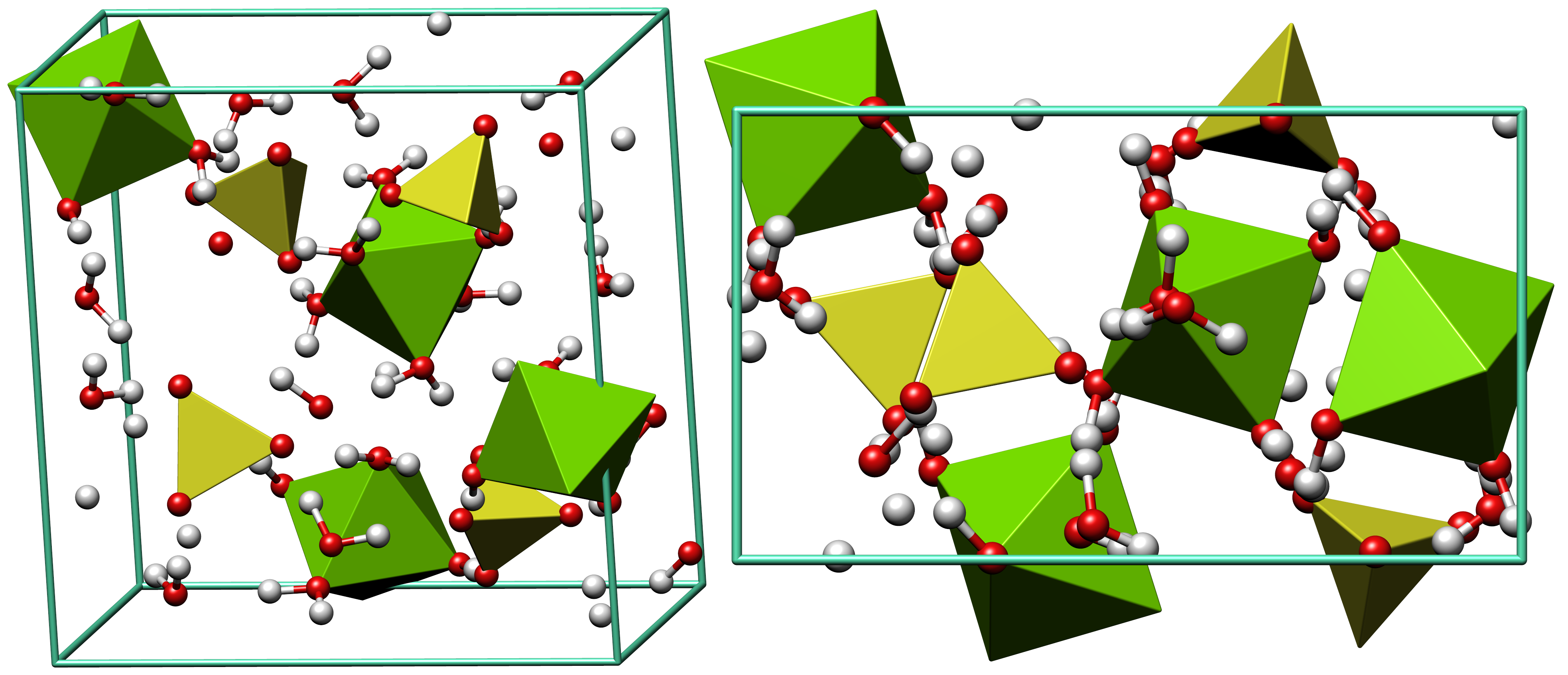
Epsomite
Epsomite, Epsom salt, or magnesium sulfate heptahydrate, is a hydrous magnesium sulfate mineral with formula MgSO4·7H2O. Epsomite crystallizes in the orthorhombic system as rarely found acicular or fibrous crystals, the normal form is as massive encrustations. It is colorless to white with tints of yellow, green and pink. The Mohs hardness is 2 to 2.5 and it has a low specific gravity of 1.67. It is readily soluble in water. It absorbs water from the air and converts to hexahydrate with the loss of one water molecule and a switch to monoclinic structure. Etymology It was first systematically described in 1806 for an occurrence near Epsom, Surrey, England, after which it was named. Discovery and occurrence Epsomite forms as encrustations or efflorescences on limestone cavern walls and mine timbers and walls, rarely as volcanic fumarole deposits, and as rare beds in evaporite layers such as those found in certain bodies of salt water. It occurs in association with melanterite, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons while an anion is a negatively charged ion with more electrons than protons. Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or polyatomic ions. In the case of physical ionization in a fluid (gas or liquid), "ion pairs" are created by spontaneous molecule collisions, where each generated pair consists of a free electron and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crystallization
Crystallization is the process by which solid forms, where the atoms or molecules are highly organized into a structure known as a crystal. Some ways by which crystals form are precipitating from a solution, freezing, or more rarely deposition directly from a gas. Attributes of the resulting crystal depend largely on factors such as temperature, air pressure, and in the case of liquid crystals, time of fluid evaporation. Crystallization occurs in two major steps. The first is nucleation, the appearance of a crystalline phase from either a supercooled liquid or a supersaturated solvent. The second step is known as crystal growth, which is the increase in the size of particles and leads to a crystal state. An important feature of this step is that loose particles form layers at the crystal's surface and lodge themselves into open inconsistencies such as pores, cracks, etc. The majority of minerals and organic molecules crystallize easily, and the resulting crystals are g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spring (water)
A spring is a point of exit at which groundwater from an aquifer flows out on top of Earth's crust (pedosphere) and becomes surface water. It is a component of the hydrosphere. Springs have long been important for humans as a source of fresh water, especially in arid regions which have relatively little annual rainfall. Springs are driven out onto the surface by various natural forces, such as gravity and hydrostatic pressure. Their yield varies widely from a volumetric flow rate of nearly zero to more than for the biggest springs. Formation Springs are formed when groundwater flows onto the surface. This typically happens when the groundwater table reaches above the surface level. Springs may also be formed as a result of karst topography, aquifers, or volcanic activity. Springs also have been observed on the ocean floor, spewing hot water directly into the ocean. Springs formed as a result of karst topography create karst springs, in which ground water travels through ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfate Mineral
The sulfate minerals are a class of minerals that include the sulfate ion () within their structure. The sulfate minerals occur commonly in primary evaporite depositional environments, as gangue minerals in hydrothermal veins and as secondary minerals in the oxidizing zone of sulfide mineral deposits. The chromate and manganate minerals have a similar structure and are often included with the sulfates in mineral classification systems.Klein, Cornelis and Cornelius S. Hurlbut, 1985, ''Manual of Mineralogy,'' 20th ed., John Wiley and Sons, New York, pp. 347–354 . Sulfate minerals include: *Anhydrous sulfates **Barite BaSO4 ** Celestite SrSO4 **Anglesite PbSO4 **Anhydrite CaSO4 **Hanksite Na22K(SO4)9(CO3)2Cl *Hydroxide and hydrous sulfates **Gypsum CaSO4·2H2O **Chalcanthite CuSO4·5H2O **Kieserite MgSO4·H2O ** Starkeyite MgSO4·4H2O **Hexahydrite MgSO4·6H2O **Epsomite MgSO4·7H2O **Meridianiite MgSO4·11H2O **Melanterite FeSO4·7H2O **Antlerite Cu3SO4(OH)4 **Brochantite Cu4SO4 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood. Chemical nature Inorganic chemistry Hydrates are inorganic salts "containing water molecules combined in a definite ratio as an integral part of the crystal" that are either bound to a metal center or that have crystallized with the metal complex. Such hydrates are also said to contain ''water of crystallization'' or ''water of hydration''. If the water is heavy water in which the constituent hydrogen is the isotope deuterium, then the term ''deuterate'' may be used in place of ''hydrate''. A colorful example is cobalt(II) chloride, which turns from blue to red upon hydration, and can therefore be used as a water indicator. The notation "''hydrated compound''⋅''n''", where ''n'' is the number of water molecules per formula un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photosynthesis
Photosynthesis is a process used by plants and other organisms to convert light energy into chemical energy that, through cellular respiration, can later be released to fuel the organism's activities. Some of this chemical energy is stored in carbohydrate molecules, such as sugars and starches, which are synthesized from carbon dioxide and water – hence the name ''photosynthesis'', from the Greek ''phōs'' (), "light", and ''synthesis'' (), "putting together". Most plants, algae, and cyanobacteria perform photosynthesis; such organisms are called photoautotrophs. Photosynthesis is largely responsible for producing and maintaining the oxygen content of the Earth's atmosphere, and supplies most of the energy necessary for life on Earth. Although photosynthesis is performed differently by different species, the process always begins when energy from light is absorbed by proteins called reaction centers that contain green chlorophyll (and other colored) pigments/chromoph ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorophyll
Chlorophyll (also chlorophyl) is any of several related green pigments found in cyanobacteria and in the chloroplasts of algae and plants. Its name is derived from the Greek words , ("pale green") and , ("leaf"). Chlorophyll allow plants to absorb energy from light. Chlorophylls absorb light most strongly in the blue portion of the electromagnetic spectrum as well as the red portion. Conversely, it is a poor absorber of green and near-green portions of the spectrum. Hence chlorophyll-containing tissues appear green because green light, diffusively reflected by structures like cell walls, is less absorbed. Two types of chlorophyll exist in the photosystems of green plants: chlorophyll ''a'' and ''b''. History Chlorophyll was first isolated and named by Joseph Bienaimé Caventou and Pierre Joseph Pelletier in 1817. The presence of magnesium in chlorophyll was discovered in 1906, and was that element's first detection in living tissue. After initial work done by German chemi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plant Nutrition
Plant nutrition is the study of the chemical elements and compounds necessary for plant growth and reproduction, plant metabolism and their external supply. In its absence the plant is unable to complete a normal life cycle, or that the element is part of some essential plant constituent or metabolite. This is in accordance with Justus von Liebig’s law of the minimum. The total essential plant nutrients include seventeen different elements: carbon, oxygen and hydrogen which are absorbed from the air, whereas other nutrients including nitrogen are typically obtained from the soil (exceptions include some parasitic or carnivorous plants). Plants must obtain the following mineral nutrients from their growing medium: * the macronutrients: nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), sulfur (S), magnesium (Mg), carbon (C), oxygen (O), hydrogen (H) * the micronutrients (or trace minerals): iron (Fe), boron (B), chlorine (Cl), manganese (Mn), zinc (Zn), copper (Cu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bath Salts
Bath salts are water-soluble, pulverized minerals that are added to water to be used for bathing. They are said to improve cleaning, enhance the enjoyment of bathing, and serve as a vehicle for cosmetic agents. Bath salts have been developed which mimic the properties of natural mineral baths or hot springs. Some bath salts contain glycerine so the product will act as an emollient, humectant, or lubricant. Fragrances and colors are often added to bath salts; the fragrances are used to increase the users' enjoyment of the bathing experience. Description Substances often labeled as bath salts include magnesium sulfate (Epsom salts), sodium chloride (table salt), sodium bicarbonate (baking soda), sodium hexametaphosphate (Calgon, amorphous/glassy sodium metaphosphate), sodium sesquicarbonate, borax, and sodium citrate. Glycerin, or liquid glycerin, is another common ingredient in bath salts. Depending on their properties, the additives can be classified as emollient, humect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Household Chemical
Household chemicals are non-food chemicals that are commonly found and used in and around the average household. They are a type of consumer goods, designed particularly to assist cleaning, house and yard maintenance, cooking, pest control and general hygiene purposes often stored in the kitchen or garage. Food additives generally do not fall under this category, unless they have a use other than for human consumption. Additives in general (e.g. stabilizers and coloring found in washing powder and dishwasher detergents) make the classification of household chemicals more complex, especially in terms of health - some of these chemicals are irritants or potent allergens - and ecological effects. Together with non-compostable household waste, the chemicals found in private household commodities pose a serious ecological problem. In addition to having slightly adverse up to seriously toxic effects when swallowed, chemical agents around may contain flammable or corrosive substances ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



_chloride.jpg)
(NRCS_Photo_Gallery).jpg)
