|
Myosin Binding Protein C, Cardiac
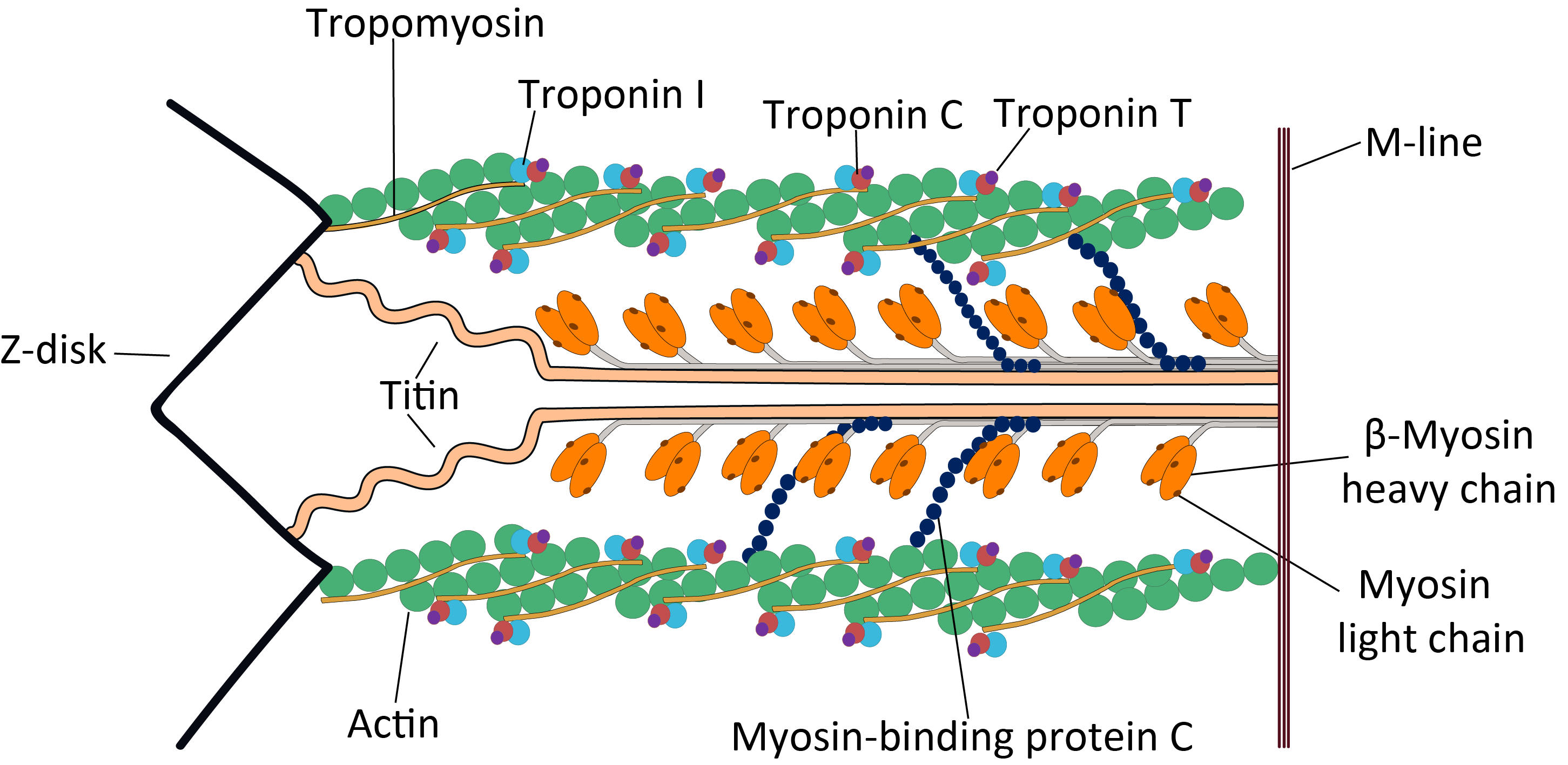
The myosin-binding protein C, cardiac-type is a protein that in humans is encoded by the ''MYBPC3'' gene. This isoform is expressed exclusively in heart muscle during human and mouse development, and is distinct from those expressed in slow skeletal muscle (MYBPC1) and fast skeletal muscle (MYBPC2). Structure cMyBP-C is a 140.5 kDa protein composed of 1273 amino acids. cMyBP-C is a myosin-associated protein that binds at 43 nm intervals along the myosin thick filament backbone, stretching for 200 nm on either side of the M-line within the crossbridge-bearing zone (C-region) of the A band in striated muscle. The approximate stoichiometry of cMyBP-C along the thick filament is 1 per 9-10 myosin molecules, or 37 cMyBP-C molecules per thick filament. In addition to myosin, cMyBP-C also binds titin and actin. The cMyBP-C isoform expressed in cardiac muscle differs from those expressed in slow and fast skeletal muscle (MYBPC1 and MYBPC2, respectively) by three features: (1) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cardiac Sarcomere Structure
The heart is a muscular organ in most animals. This organ pumps blood through the blood vessels of the circulatory system. The pumped blood carries oxygen and nutrients to the body, while carrying metabolic waste such as carbon dioxide to the lungs. In humans, the heart is approximately the size of a closed fist and is located between the lungs, in the middle compartment of the chest. In humans, other mammals, and birds, the heart is divided into four chambers: upper left and right atria and lower left and right ventricles. Commonly the right atrium and ventricle are referred together as the right heart and their left counterparts as the left heart. Fish, in contrast, have two chambers, an atrium and a ventricle, while most reptiles have three chambers. In a healthy heart blood flows one way through the heart due to heart valves, which prevent backflow. The heart is enclosed in a protective sac, the pericardium, which also contains a small amount of fluid. The wal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Kinases
A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them (phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a functional change of the target protein ( substrate) by changing enzyme activity, cellular location, or association with other proteins. The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. There are two main types of protein kinase. The great majority are serine/threonine kinases, which phosphorylate the hydroxyl groups of serines and threonines in their targets and most of the others are tyrosine kinases, although additional types exist. Protein kinases are also found in bacteria and plants. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction. Chemical act ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Troponin T
Troponin T (shortened TnT or TropT) is a part of the troponin complex, which are proteins integral to the contraction of skeletal and heart muscles. They are expressed in skeletal and cardiac myocytes. Troponin T binds to tropomyosin and helps position it on actin, and together with the rest of the troponin complex, modulates contraction of striated muscle. The cardiac subtype of troponin T is especially useful in the laboratory diagnosis of heart attack because it is released into the blood-stream when damage to heart muscle occurs. It was discovered by the German physician Hugo A. Katus at the University of Heidelberg, who also developed the troponin T assay. Subtypes * Slow skeletal troponin T1, TNNT1 (19q13.4, ) * Cardiac troponin T2, TNNT2 (1q32, ) * Fast skeletal troponin T3, TNNT3 (11p15.5, ) Reference values The 99th percentile cutoff for cardiac troponin T (cTnT) is 0.01 ng/mL. The reference range for the high sensitivity troponin T is a normal 52 ng/L. Background ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TPM1
Tropomyosin alpha-1 chain is a protein that in humans is encoded by the ''TPM1'' gene. This gene is a member of the tropomyosin (Tm) family of highly conserved, widely distributed actin-binding proteins involved in the contractile system of striated and smooth muscles and the cytoskeleton of non-muscle cells. Structure Tm is a 32.7 kDa protein composed of 284 amino acids. Tm is a flexible protein homodimer or heterodimer composed of two alpha-helical chains, which adopt a bent coiled coil conformation to wrap around the seven actin molecules in a functional unit of muscle. It is polymerized end to end along the two grooves of actin filaments and provides stability to the filaments. Human striated muscles express protein from the ''TPM1'' (α-Tm), ''TPM2'' (β-Tm) and '' TPM3'' (γ-Tm) genes, with α-Tm being the predominant isoform in striated muscle. In human cardiac muscle the ratio of α-Tm to β-Tm is roughly 5:1. Function Tm functions in association with the troponin complex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TNNT2
Cardiac muscle troponin T (cTnT) is a protein that in humans is encoded by the ''TNNT2'' gene. Cardiac TnT is the tropomyosin-binding subunit of the troponin complex, which is located on the thin filament of striated muscles and regulates muscle contraction in response to alterations in intracellular calcium ion concentration. The TNNT2 gene is located at 1q32 in the human chromosomal genome, encoding the cardiac muscle isoform of troponin T (cTnT). Human cTnT is an ~36-kDa protein consisting of 297 amino acids including the first methionine with an isoelectric point (pI) of 4.88. It is the tropomyosin- binding and thin filament anchoring subunit of the troponin complex in cardiac muscle cells. TNNT2 gene is expressed in vertebrate cardiac muscles and embryonic skeletal muscles. Structure Cardiac TnT is a 35.9 kDa protein composed of 298 amino acids. Cardiac TnT is the largest of the three troponin subunits (cTnT, troponin I (TnI), troponin C (TnC)) on the actin thin filament o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myosin Heavy Chain
Myosins () are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility. The first myosin (M2) to be discovered was in 1864 by Wilhelm Kühne. Kühne had extracted a viscous protein from skeletal muscle that he held responsible for keeping the tension state in muscle. He called this protein ''myosin''. The term has been extended to include a group of similar ATPases found in the cells of both striated muscle tissue and smooth muscle tissue. Following the discovery in 1973 of enzymes with myosin-like function in '' Acanthamoeba castellanii'', a global range of divergent myosin genes have been discovered throughout the realm of eukaryotes. Although myosin was originally thought to be restricted to muscle cells (hence '' myo-''(s) + '' -in''), there is no single "myosin"; rather it is a very large superfamily of genes whose prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MYH7
MYH7 is a gene encoding a myosin heavy chain beta (MHC-β) isoform (slow twitch) expressed primarily in the heart, but also in skeletal muscles (type I fibers). This isoform is distinct from the fast isoform of cardiac myosin heavy chain, MYH6, referred to as MHC-α. MHC-β is the major protein comprising the thick filament in cardiac muscle and plays a major role in cardiac muscle contraction. Structure MHC-β is a 223 kDa protein composed of 1935 amino acids. MHC-β is a hexameric, asymmetric motor forming the bulk of the thick filament in cardiac muscle. MHC-β is composed of N-terminal globular heads (20 nm) that project laterally, and alpha helical tails (130 nm) that dimerize and multimerize into a coiled-coil motif to form the light meromyosin (LMM), thick filament rod. The 9 nm alpha-helical neck region of each MHC-β head non-covalently binds two light chains, essential light chain (MYL3) and regulatory light chain (MYL2). Approximately 300 myosin molecu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
GSK3β
Glycogen synthase kinase-3 beta, (GSK-3 beta), is an enzyme that in humans is encoded by the ''GSK3B'' gene. In mice, the enzyme is encoded by the Gsk3b gene. Abnormal regulation and expression of GSK-3 beta is associated with an increased susceptibility towards bipolar disorder. Function Glycogen synthase kinase-3 (GSK-3) is a proline-directed serine-threonine kinase that was initially identified as a phosphorylating and an inactivating agent of glycogen synthase. Two isoforms, alpha (GSK3A) and beta, show a high degree of amino acid homology. GSK3B is involved in energy metabolism, neuronal cell development, and body pattern formation. It might be a new therapeutic target for ischemic stroke. Disease relevance Homozygous disruption of the Gsk3b locus in mice results in embryonic lethality during mid-gestation. This lethality phenotype could be rescued by inhibition of tumor necrosis factor. Two SNPs at this gene, rs334558 (-50T/C) and rs3755557 (-1727A/T), are associat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Kinase C
In cell biology, Protein kinase C, commonly abbreviated to PKC (EC 2.7.11.13), is a family of protein kinase enzymes that are involved in controlling the function of other proteins through the phosphorylation of hydroxyl groups of serine and threonine amino acid residues on these proteins, or a member of this family. PKC enzymes in turn are activated by signals such as increases in the concentration of diacylglycerol (DAG) or calcium ions (Ca2+). Hence PKC enzymes play important roles in several signal transduction cascades. In biochemistry, the PKC family consists of fifteen isozymes in humans. They are divided into three subfamilies, based on their second messenger requirements: conventional (or classical), novel, and atypical. Conventional (c)PKCs contain the isoforms α, βI, βII, and γ. These require Ca2+, DAG, and a phospholipid such as phosphatidylserine for activation. Novel (n)PKCs include the δ, ε, η, and θ isoforms, and require DAG, but do not require Ca2+ for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Kinase D
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ribosomal S6 Kinase
In molecular biology, ribosomal s6 kinase (rsk) is a family of protein kinases involved in signal transduction. There are two subfamilies of rsk, p90rsk, also known as MAPK-activated protein kinase-1 (MAPKAP-K1), and p70rsk, also known as S6-H1 Kinase or simply S6 Kinase. There are three variants of p90rsk in humans, rsk 1-3. Rsks are serine/threonine kinases and are activated by the MAPK/ERK pathway. There are two known mammalian homologues of S6 Kinase: S6K1 and S6K2. Substrates Both p90 and p70 Rsk phosphorylate ribosomal protein s6, part of the translational machinery, but several other substrates have been identified, including other ribosomal proteins. Cytosolic substrates of p90rsk include protein phosphatase 1; glycogen synthase kinase 3 (GSK3); L1 CAM, a neural cell adhesion molecule; Son of Sevenless, the Ras exchange factor; and Myt1, an inhibitor of cdc2. RSK phosphorylation of SOS1 (Son of Sevenless) at Serines 1134 and 1161 creates 14-3-3 docking site. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CaMKII
/calmodulin-dependent protein kinase II (CaM kinase II or CaMKII) is a serine/threonine-specific protein kinase that is regulated by the / calmodulin complex. CaMKII is involved in many signaling cascades and is thought to be an important mediator of learning and memory. CaMKII is also necessary for homeostasis and reuptake in cardiomyocytes, chloride transport in epithelia, positive T-cell selection, and CD8 T-cell activation. Misregulation of CaMKII is linked to Alzheimer's disease, Angelman syndrome, and heart arrhythmia. Types There are two types of CaM kinase: * Specialized CaM kinases, such as the myosin light chain kinase that phosphorylates myosin, causing smooth muscles to contract * Multifunctional CaM kinases, also collectively called ''CaM kinase II'', which play a role in neurotransmitter secretion, transcription factor regulation, and glycogen metabolism. Structure, function, and autoregulation CaMKII accounts for 1–2% of all proteins in the brain ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |