TNNT2 on:
[Wikipedia]
[Google]
[Amazon]
 Cardiac muscle troponin T (cTnT) is a
Cardiac muscle troponin T (cTnT) is a
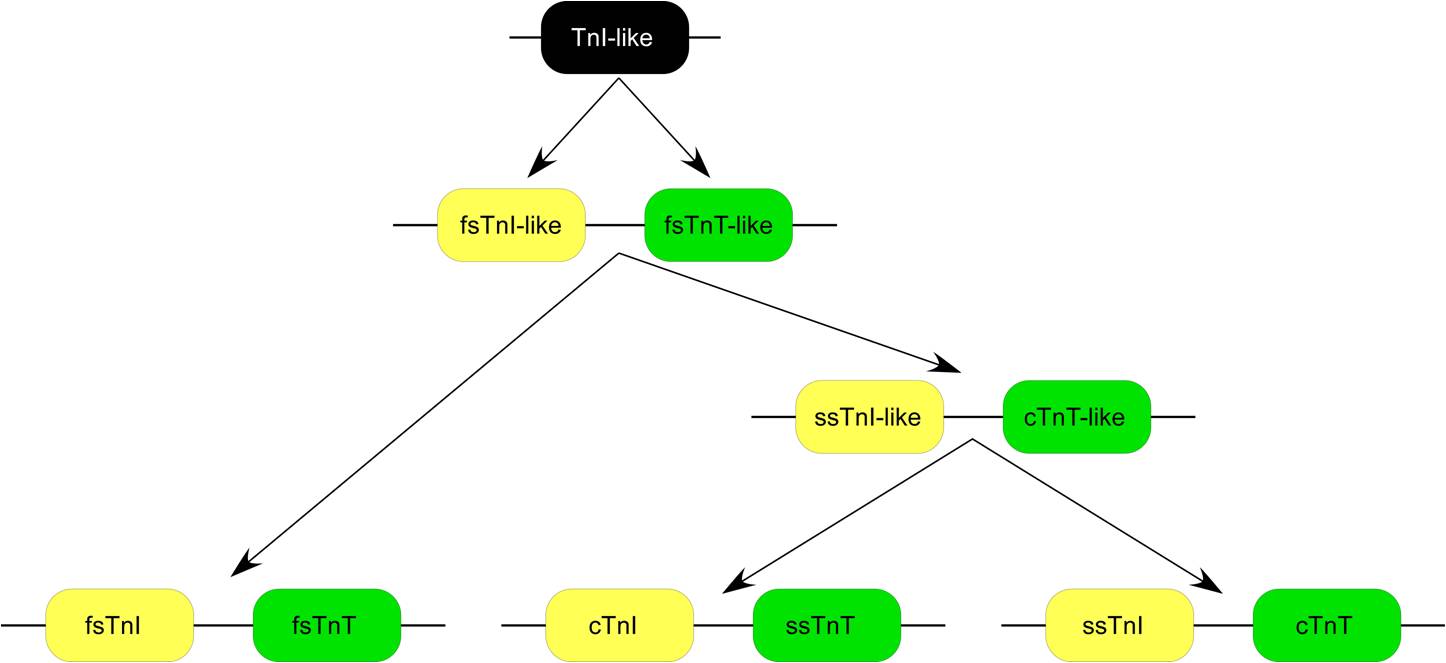 Three homologous genes have evolved in vertebrates encoding three muscle type- specific isoforms of TnT. Each of the TnT isoform genes is linked in chromosomal DNA to a troponin I (TnI) isoform gene encoding the inhibitory subunit of the troponin complex to form three gene pairs: The fast skeletal muscle TnI (fsTnI)-fsTnT, slow skeletal muscle TnI (ssTnI)-cTnT, and cTnI-ssTnT pairs. Sequence and epitope conservation studies suggested that genes encoding the muscle type-specific TnT and TnI isoforms have originated from a TnI-like ancestor gene and duplicated and diversified from a fsTnI-like-fsTnT-like gene pair.
231x231px
The apparently scrambled linkage between ssTnI-cTnT and cTnI-ssTnT genes actually reflects original functional linkages as that TNNT2 gene is expressed together with ssTnI gene in embryonic cardiac muscle. Protein sequence alignment demonstrated that TNNT2 gene is conserved in vertebrate species (Fig. 2) in the middle and C-terminal regions, while the three muscle type isoforms are significantly diverged.
Three homologous genes have evolved in vertebrates encoding three muscle type- specific isoforms of TnT. Each of the TnT isoform genes is linked in chromosomal DNA to a troponin I (TnI) isoform gene encoding the inhibitory subunit of the troponin complex to form three gene pairs: The fast skeletal muscle TnI (fsTnI)-fsTnT, slow skeletal muscle TnI (ssTnI)-cTnT, and cTnI-ssTnT pairs. Sequence and epitope conservation studies suggested that genes encoding the muscle type-specific TnT and TnI isoforms have originated from a TnI-like ancestor gene and duplicated and diversified from a fsTnI-like-fsTnT-like gene pair.
231x231px
The apparently scrambled linkage between ssTnI-cTnT and cTnI-ssTnT genes actually reflects original functional linkages as that TNNT2 gene is expressed together with ssTnI gene in embryonic cardiac muscle. Protein sequence alignment demonstrated that TNNT2 gene is conserved in vertebrate species (Fig. 2) in the middle and C-terminal regions, while the three muscle type isoforms are significantly diverged.
Mass spectrometry characterization of human TNNT2 at COPaKB
GeneReviews/NIH/NCBI/UW entry on Familial Hypertrophic Cardiomyopathy Overview
* {{Cytoskeletal Proteins
 Cardiac muscle troponin T (cTnT) is a
Cardiac muscle troponin T (cTnT) is a protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, res ...
that in humans is encoded by the ''TNNT2'' gene
In biology, the word gene (from , ; "... Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a b ...
. Cardiac TnT is the tropomyosin
Tropomyosin is a two-stranded alpha-helical, coiled coil protein found in actin-based cytoskeletons.
Tropomyosin and the actin skeleton
All organisms contain organelles that provide physical integrity to their cells. These type of organelles a ...
-binding subunit of the troponin
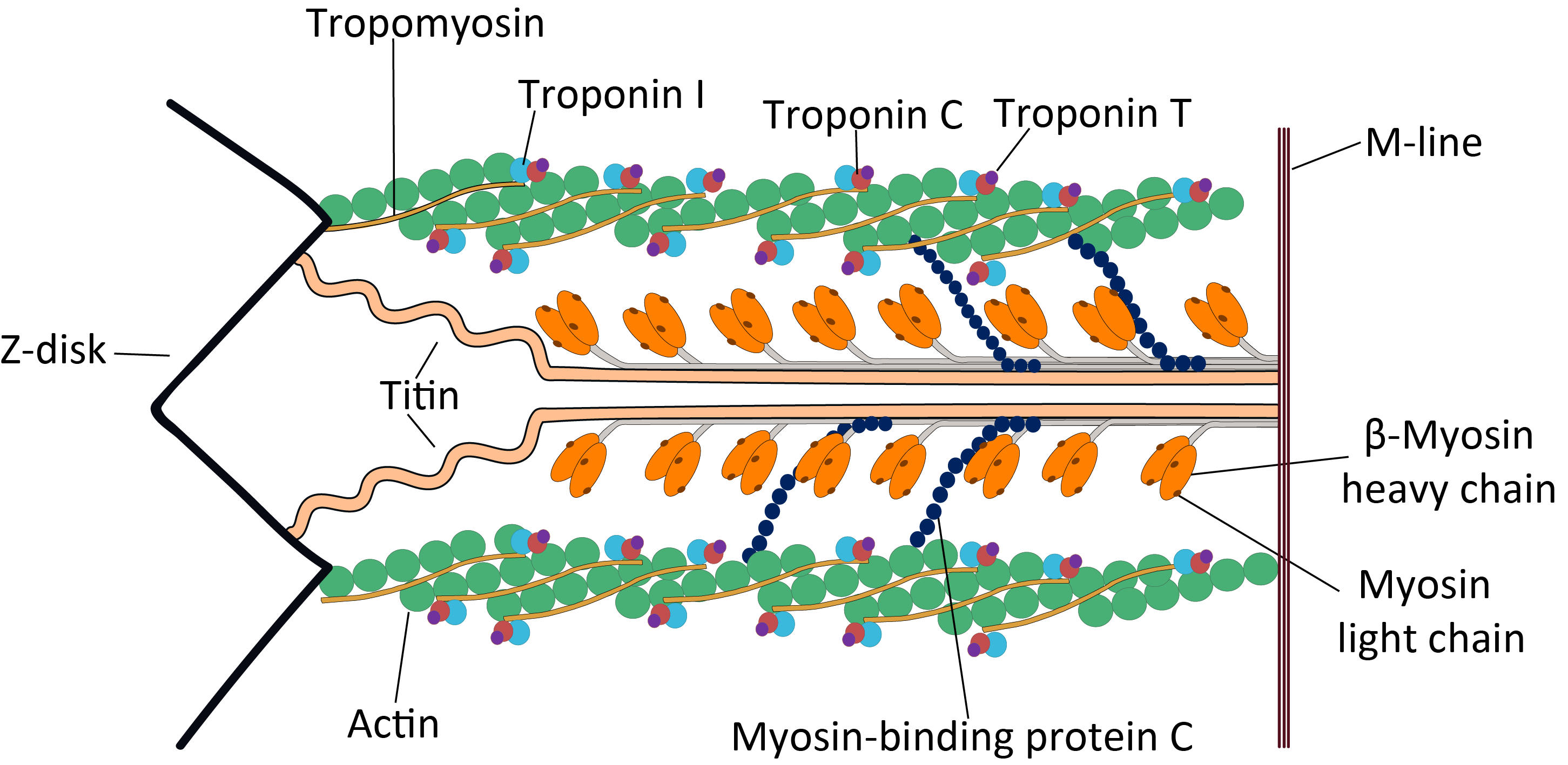
image:Troponin Ribbon Diagram.png, 400px, Ribbon representation of the human cardiac troponin core complex (52 kDa core) in the calcium-saturated form. Blue = troponin C; green = troponin I; magenta = troponin T.; ; rendered with PyMOL
Troponin, ...
complex, which is located on the thin filament of striated muscles and regulates muscle contraction in response to alterations in intracellular calcium ion concentration.
The TNNT2 gene is located at 1q32 in the human chromosomal genome, encoding the cardiac muscle isoform of troponin T (cTnT). Human cTnT is an ~36-kDa protein consisting of 297 amino acids including the first methionine with an isoelectric point (pI) of 4.88. It is the tropomyosin- binding and thin filament anchoring subunit of the troponin complex in cardiac muscle cells. TNNT2 gene is expressed in vertebrate cardiac muscles and embryonic skeletal muscles.
Structure
Cardiac TnT is a 35.9 kDa protein composed of 298 amino acids. Cardiac TnT is the largest of the three troponin subunits (cTnT,troponin I
Troponin I is a cardiac and skeletal muscle protein family. It is a part of the troponin protein complex, where it binds to actin in thin myofilaments to hold the actin-tropomyosin complex in place. Troponin I prevents myosin from binding to ac ...
(TnI), troponin C
Troponin C is a protein which is part of the troponin complex. It contains four calcium-binding EF hands, although different isoforms may have fewer than four functional calcium-binding subdomains. It is a component of thin filaments, along wi ...
(TnC)) on the actin
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of ov ...
thin filament of cardiac muscle. The structure of TnT is asymmetric; the globular C-terminal domain interacts with tropomyosin
Tropomyosin is a two-stranded alpha-helical, coiled coil protein found in actin-based cytoskeletons.
Tropomyosin and the actin skeleton
All organisms contain organelles that provide physical integrity to their cells. These type of organelles a ...
(Tm), TnI and TnC, and the N-terminal tether which strongly binds Tm. The N-terminal region of TnT is alternatively spliced, accounting for multiple isoforms observed in cardiac muscle.
Function
As part of the Troponin complex, the function of cTnT is to regulate muscle contraction. The N-terminal region of TnT that strongly bindsactin
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of ov ...
most likely moves with Tm and actin
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of ov ...
during strong myosin crossbridge binding and force generation. This region is likely involved in the transduction of cooperativity down the thin filament. The C-terminal region of TnT constitutes part of the globular troponin complex domain, and participates in employing the calcium sensitivity of strong myosin crossbridge binding to the thin filament.
Clinical significance
Mutations in this gene have been associated with familial hypertrophic cardiomyopathy as well as with restrictive anddilated cardiomyopathy
Dilated cardiomyopathy (DCM) is a condition in which the heart becomes enlarged and cannot pump blood effectively. Symptoms vary from none to feeling tired, leg swelling, and shortness of breath. It may also result in chest pain or fainting. Co ...
. Transcripts for this gene undergo alternative splicing that results in many tissue-specific isoforms, however, the full-length nature of some of these variants has not yet been determined. Mutations of this gene may be associated with mild or absent hypertrophy
Hypertrophy is the increase in the volume of an organ or tissue due to the enlargement of its component cells. It is distinguished from hyperplasia, in which the cells remain approximately the same size but increase in number.Updated by Linda J. ...
and predominant restrictive disease, with a high risk of sudden cardiac death
Cardiac arrest is when the heart suddenly and unexpectedly stops beating. It is a medical emergency that, without immediate medical intervention, will result in sudden cardiac death within minutes. Cardiopulmonary resuscitation (CPR) and possib ...
. Advancement to dilated cardiomyopathy may be more rapid in patients with TNNT2 mutations than in those with myosin heavy chain
Myosins () are a superfamily of motor proteins best known for their roles in muscle contraction and in a wide range of other motility processes in eukaryotes. They are ATP-dependent and responsible for actin-based motility.
The first myosin (M2 ...
mutations.
In patients with active chronic non-inflammatory myopathy and myositis
Myositis is a rare disease that involves inflammation of the muscles. This can present with a variety of symptoms such as skin involvement (i.e., rashes), muscle weakness, and other organ involvement. Systemic symptoms such as weight loss, fatigue ...
, skeletal muscles are a significant source of cardiac troponin T without any cardiac involvement. It is advised to measure cardiac troponin I instead if a skeletal muscle disorder is suspected.
;Elevated levels after Covid-19 mRNA vaccinations
A study carried out by the University of Basel
The University of Basel (Latin: ''Universitas Basiliensis'', German: ''Universität Basel'') is a university in Basel, Switzerland. Founded on 4 April 1460, it is Switzerland's oldest university and among the world's oldest surviving universit ...
and the University Hospital of Basel
The University Hospital of Basel (German: ''Universitätsspital Basel'', USB), in Basel, is one of the five university hospitals of Switzerland. Since 1842 it is located in a former palace, the Markgräflerhof.
USB brings together 50 clinics, ...
found that a Covid-19
Coronavirus disease 2019 (COVID-19) is a contagious disease caused by a virus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The first known case was identified in Wuhan, China, in December 2019. The disease quickly ...
mRNA vaccination significantly elevates the cardiac troponin T levels in the blood stream. 3 % of the study subjects have shown elevated amounts of the protein after their 3rd vaccination. The effect was most pronounced among young men. It is not yet clear what the mechanism is, and the observed troponin levels were still much lower than in clinically significant heart disease. Given that previous studies only registered 35 cases of heart muscle inflammation per million subjects, the involved researchers were surprised by the results.
Evolution
 Three homologous genes have evolved in vertebrates encoding three muscle type- specific isoforms of TnT. Each of the TnT isoform genes is linked in chromosomal DNA to a troponin I (TnI) isoform gene encoding the inhibitory subunit of the troponin complex to form three gene pairs: The fast skeletal muscle TnI (fsTnI)-fsTnT, slow skeletal muscle TnI (ssTnI)-cTnT, and cTnI-ssTnT pairs. Sequence and epitope conservation studies suggested that genes encoding the muscle type-specific TnT and TnI isoforms have originated from a TnI-like ancestor gene and duplicated and diversified from a fsTnI-like-fsTnT-like gene pair.
231x231px
The apparently scrambled linkage between ssTnI-cTnT and cTnI-ssTnT genes actually reflects original functional linkages as that TNNT2 gene is expressed together with ssTnI gene in embryonic cardiac muscle. Protein sequence alignment demonstrated that TNNT2 gene is conserved in vertebrate species (Fig. 2) in the middle and C-terminal regions, while the three muscle type isoforms are significantly diverged.
Three homologous genes have evolved in vertebrates encoding three muscle type- specific isoforms of TnT. Each of the TnT isoform genes is linked in chromosomal DNA to a troponin I (TnI) isoform gene encoding the inhibitory subunit of the troponin complex to form three gene pairs: The fast skeletal muscle TnI (fsTnI)-fsTnT, slow skeletal muscle TnI (ssTnI)-cTnT, and cTnI-ssTnT pairs. Sequence and epitope conservation studies suggested that genes encoding the muscle type-specific TnT and TnI isoforms have originated from a TnI-like ancestor gene and duplicated and diversified from a fsTnI-like-fsTnT-like gene pair.
231x231px
The apparently scrambled linkage between ssTnI-cTnT and cTnI-ssTnT genes actually reflects original functional linkages as that TNNT2 gene is expressed together with ssTnI gene in embryonic cardiac muscle. Protein sequence alignment demonstrated that TNNT2 gene is conserved in vertebrate species (Fig. 2) in the middle and C-terminal regions, while the three muscle type isoforms are significantly diverged.
Alternative splicing
Mammalian TNNT2 gene contains 14 constitutive exons and 3 alternatively spliced exons. Exons 4 and 5 encoding the N-terminal variable region and exon 13 between the middle and C-terminal regions are alternatively spliced. Exon 5 encodes a 9 or 10 amino acid segment that is highly acidic and negatively charged at physiological pH. Exon 5 is expressed in embryonic heart, down-regulated and ceases express during postnatal development. Embryonic cTnT with more negative charge at the N-terminal region exerts higher calcium sensitivity of actomyosin ATPase activity and myofilament force production, compared with the adult cardiac TnT, as well as a higher tolerance to acidosis. TNNT2 gene is transiently expressed in embryonic and neonatal skeletal muscles in both avian and mammalian organisms. When TNNT2 is expressed in neonatal skeletal muscle, the alternative splicing of exon 5 exhibits a synchronized regulation to that in the heart in a species-specific manner. This phenomenon indicates that alternative splicing of TNNT2 pre-mRNA is under the control of a genetically built- in systemic biological clock.Posttranslational modifications
Phosphorylation
Ser2 of cTnT at the N terminus is constitutively phosphorylated by unknown mechanisms. cTnT has been found to be phosphorylated by PKC at Thr197, Ser201, Thr206, Ser208 and Thr287 in the C-terminal region. Phosphorylation of Thr206 alone was sufficient to reduce myofilament calcium sensitivity and force production. cTnT is also phosphorylated at Thr194 and Ser198 under stress conditions, leading to attenuated cardiomyocyte contractility. Phosphorylation of cTnT at Ser278 and Thr287 by ROCK-II was shown to decrease myosin ATPase activity and myofilament force development in skinned cardiac muscle. Table 1 summarizes the phosphorylation modifications of cTnT and possible functions.O-linked GlcNAcylation
cTnT is increasingly modified at Ser190 by O-GlcNAcylation during the development of heart failure in rat, accompanied by decreased phosphorylation of Ser208.Proteolytic modification
In apoptotic cardiomyocytes, cTnT was cleaved by caspase 3 to generate a 25-kDa N-terminal truncated fragment. This destructive fragmentation removes a part of the middle region tropomyosin binding site 1, leading to attenuation of the myofilament force production by decreasing the myosin ATPase activity. In cardiac muscle under stress conditions, cardiac TnT is cleaved by calpain I, restrictively removing the entire N-terminal variable region. This proteolytic modification of cTnT occurs in cardiac muscle in acute ischemia-reperfusion or pressure overload. The restrictively N-terminal truncated cTnT remains functional in the myofilaments and leads to reduced contractile velocity of the ventricular muscle, which extends the rapid ejection phase and results in an increase in stroke volume, especially under increased afterload. In vitro studies showed that N-terminal truncated cTnT preserved the overall cardiac myofilament calcium sensitivity and cooperativity, but altered TnT's binding affinities for tropomyosin, TnI and TnC proteins, and lead to slightly decreased maximum myosin ATPase activity and myofilament force production, which forms the basis of the selective decrease in contractile velocity of ventricular muscle to increase stroke volume without significant increase in energy expenditure. With the relatively short half life of cTnT in cardiomyocytes (3–4 days), the N-terminal truncated cTnT would be replaced by newly synthesized intact cTnT in several days. Therefore, this mechanism provides a reversible posttranslational regulation to modulate cardiac function in adaptation to stress conditions. The residues in cardiac TnT with phosphorylation regulations are summarized. The residue numbers for phosphorylatable serine and threonine are that in human cardiac TnT with the first methionine included. The phosphorylation of cardiac TnT at these residues is compared with the counterparts in fast TnT and slow TnT. C, conserved; N, non-conserved. Kinases responsible for each phosphorylation, functional effects, and references are also listed.Mutations in cardiomyopathies
Point mutations in TNNT2 gene cause various types of cardiomyopathies, including hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM) and restrictive cardiomyopathy (RCM). The table below summarizes representative TNNT2 mutations and abnormal splicings found in human and animal cardiomyopathies. Amino Acid residues of mutations were numbered as in human cardiac TnT with the first methionine included. Mutations of cardiac TnT that caused cardiomyopathies were mostly found in the conserved middle and C-terminal regions.Notes
References
External links
Mass spectrometry characterization of human TNNT2 at COPaKB
GeneReviews/NIH/NCBI/UW entry on Familial Hypertrophic Cardiomyopathy Overview
* {{Cytoskeletal Proteins