|
Methyl Nitrate
Methyl nitrate is the methyl ester of nitric acid and has the chemical formula CH3NO3. It is a colourless explosive volatile liquid. Synthesis It can be produced by the condensation of nitric acid and methanol: :CH3OH + HNO3 → CH3NO3 + H2O A newer method uses methyl iodide and silver nitrate: CH3I + AgNO3 → CH3NO3 + AgI Methyl nitrate can be produced on a laboratory or industrial scale either through the distillation of a mixture of methanol and nitric acid, or by the nitration of methanol by a mixture of sulfuric and nitric acids. The first procedure is not preferred due to the great explosion danger presented by the methyl nitrate vapour. The second procedure is essentially identical to that of making nitroglycerin. However, the process is usually run at a slightly higher temperature and the mixture is stirred mechanically on an industrial scale instead of with compressed air. Explosive properties Methyl nitrate is a sensitive explosive. When ignited it burns extremel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ester
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils. Esters typically have a pleasant smell; those of low molecular weight are commonly used as fragrances and are found in essential oils and pheromones. They perform as high-grade solvents for a broad array of plastics, plasticizers, resins, and lacquers, and are one of the largest classes of synthetic lubricants on the commercial market. Polyesters are important plastics, with monomers linked by ester moieties. Phosphoesters form the backbone of DNA molecules. Nitrate esters, such as nitroglycerin, are known for their explosive properties. '' Nomenclature Etymology Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Volatility (chemistry)
In chemistry, volatility is a material quality which describes how readily a substance vaporizes. At a given temperature and pressure, a substance with high volatility is more likely to exist as a vapour, while a substance with low volatility is more likely to be a liquid or solid. Volatility can also describe the tendency of a vapor to condense into a liquid or solid; less volatile substances will more readily condense from a vapor than highly volatile ones. Differences in volatility can be observed by comparing how fast substances within a group evaporate (or sublimate in the case of solids) when exposed to the atmosphere. A highly volatile substance such as rubbing alcohol (isopropyl alcohol) will quickly evaporate, while a substance with low volatility such as vegetable oil will remain condensed. In general, solids are much less volatile than liquids, but there are some exceptions. Solids that sublimate (change directly from solid to vapor) such as dry ice (solid carbon dioxi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information. Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic, and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among various mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microwave Spectroscopy
Microwave spectroscopy is the spectroscopy method that employs microwaves, i.e. electromagnetic radiation at GHz frequencies, for the study of matter. History The ammonia molecule NH3 is shaped like a pyramid 0.38 Å in height, with an equilateral triangle of hydrogens forming the base.The nitrogen situated on the axis has two equivalent equilibrium positions above and below the triangle of hydrogens, and this raises the possibility of the nitrogen tunneling up and down, through the plane of the H-atoms. In 1932 Dennison et al. ... analyzed the vibrational energy of this molecule and concluded that the vibrational energy would be split into pairs by the presence of these two equilibrium positions. The next year Wright and Randall observed ... a splitting of 0.67 cm–1 in far infrared lines, corresponding to ν = 20 GHz, the value predicted by theory.In 1934 Cleeton and Williams ... constructed a grating echelette spectrometer in order to measure this splitting directly, thereb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Electron Diffraction
Gas electron diffraction (GED) is one of the applications of electron diffraction techniques. The target of this method is the determination of the structure of gaseous molecules, i.e., the geometrical arrangement of the atoms from which a molecule is built up. GED is one of two experimental methods (besides microwave spectroscopy) to determine the structure of free molecules, undistorted by intermolecular forces, which are omnipresent in the solid and liquid state. The determination of accurate molecular structures by GED studies is fundamental for an understanding of structural chemistry. Introduction Diffraction occurs because the wavelength of electrons accelerated by a potential of a few thousand volts is of the same order of magnitude as internuclear distances in molecules. The principle is the same as that of other electron diffraction methods such as LEED and RHEED, but the obtainable diffraction pattern is considerably weaker than those of LEED and RHEED because the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Early New High German
Early New High German (ENHG) is a term for the period in the history of the German language generally defined, following Wilhelm Scherer, as the period 1350 to 1650. The term is the standard translation of the German (Fnhd., Frnhd.), introduced by Scherer. The term ''Early Modern High German'' is also occasionally used for this period (but the abbreviation EMHG is generally used for '' Early Middle High German''). Periodisation The start and end dates of ENHG are, like all linguistic periodisations, somewhat arbitrary. In spite of many alternative suggestions, Scherer's dates still command widespread acceptance. Linguistically, the mid-14th century is marked by the phonological changes to the vowel system that characterise the modern standard language; the mid-17th sees the loss of status for regional forms of language, and the triumph of German over Latin as the dominant, and then sole, language for public discourse. Scherer's dates also have the merit of coinciding with two ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Augsburg
Augsburg (; bar , Augschburg , links=https://en.wikipedia.org/wiki/Swabian_German , label=Swabian German, , ) is a city in Swabia, Bavaria, Germany, around west of Bavarian capital Munich. It is a university town and regional seat of the ''Regierungsbezirk'' Schwaben with an impressive Altstadt (historical city centre). Augsburg is an urban district and home to the institutions of the Landkreis Augsburg. It is the third-largest city in Bavaria (after Munich and Nuremberg) with a population of 300,000 inhabitants, with 885,000 in its metropolitan area. After Neuss, Trier, Cologne and Xanten, Augsburg is one of Germany's oldest cities, founded in 15 BC by the Romans as Augsburg#Early history, Augusta Vindelicorum, named after the Roman emperor Augustus. It was a Free Imperial City from 1276 to 1803 and the home of the patrician (post-Roman Europe), patrician Fugger and Welser families that dominated European banking in the 16th century. According to Behringer, in the sixteen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
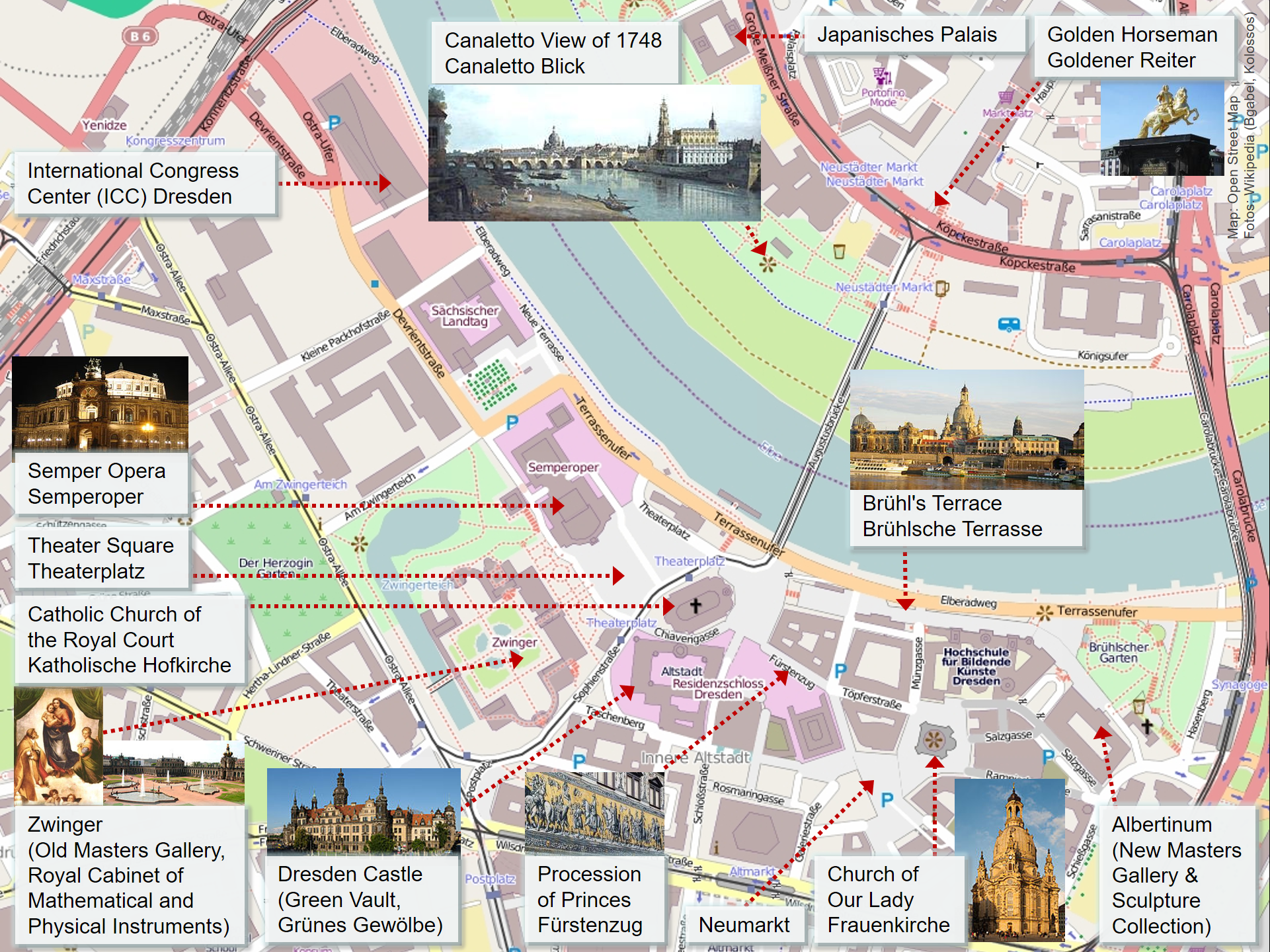
Dresden
Dresden (, ; Upper Saxon: ''Dräsdn''; wen, label=Upper Sorbian, Drježdźany) is the capital city of the German state of Saxony and its second most populous city, after Leipzig. It is the 12th most populous city of Germany, the fourth largest by area (after Berlin, Hamburg and Cologne), and the third most populous city in the area of former East Germany, after Berlin and Leipzig. Dresden's urban area comprises the towns of Freital, Pirna, Radebeul, Meissen, Coswig, Radeberg and Heidenau and has around 790,000 inhabitants. The Dresden metropolitan area has approximately 1.34 million inhabitants. Dresden is the second largest city on the River Elbe after Hamburg. Most of the city's population lives in the Elbe Valley, but a large, albeit very sparsely populated area of the city east of the Elbe lies in the West Lusatian Hill Country and Uplands (the westernmost part of the Sudetes) and thus in Lusatia. Many boroughs west of the Elbe lie in the foreland of the Ore Mounta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
German Language
German ( ) is a West Germanic languages, West Germanic language mainly spoken in Central Europe. It is the most widely spoken and Official language, official or co-official language in Germany, Austria, Switzerland, Liechtenstein, and the Italy, Italian province of South Tyrol. It is also a co-official language of Luxembourg and German-speaking Community of Belgium, Belgium, as well as a national language in Namibia. Outside Germany, it is also spoken by German communities in France (Bas-Rhin), Czech Republic (North Bohemia), Poland (Upper Silesia), Slovakia (Bratislava Region), and Hungary (Sopron). German is most similar to other languages within the West Germanic language branch, including Afrikaans, Dutch language, Dutch, English language, English, the Frisian languages, Low German, Luxembourgish, Scots language, Scots, and Yiddish. It also contains close similarities in vocabulary to some languages in the North Germanic languages, North Germanic group, such as Danish lan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reichstag Fire
The Reichstag fire (german: Reichstagsbrand, ) was an arson attack on the Reichstag building, home of the German parliament in Berlin, on Monday 27 February 1933, precisely four weeks after Nazi leader Adolf Hitler was sworn in as Chancellor of Germany. Marinus van der Lubbe, a Dutch "council communist", was the apparent culprit; however, Hitler attributed the fire to Communist agitators. He used it as a pretext to claim that Communists were plotting against the German government, and induced President Paul von Hindenburg to issue the Reichstag Fire Decree suspending civil liberties, and pursue a "ruthless confrontation" with the Communists. This made the fire pivotal in the establishment of Nazi Germany. The first report of the fire came shortly after 9:00p.m., when a Berlin fire station received an alarm call. By the time police and firefighters arrived, the Chamber of Deputies (the lower house) was engulfed in flames. The police conducted a thorough search inside the building ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Evaporation
Evaporation is a type of vaporization that occurs on the surface of a liquid as it changes into the gas phase. High concentration of the evaporating substance in the surrounding gas significantly slows down evaporation, such as when humidity affects rate of evaporation of water. When the molecules of the liquid collide, they transfer energy to each other based on how they collide. When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation occurs, the energy removed from the vaporized liquid will reduce the temperature of the liquid, resulting in evaporative cooling. On average, only a fraction of the molecules in a liquid have enough heat energy to escape from the liquid. The evaporation will continue until an equilibrium is reached when the evaporation of the liquid is equal to its condensation. In an enclosed environment, a liquid will evaporate until the surrounding air is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |





_1982%2C_MiNr_Block_068.jpg)
