|
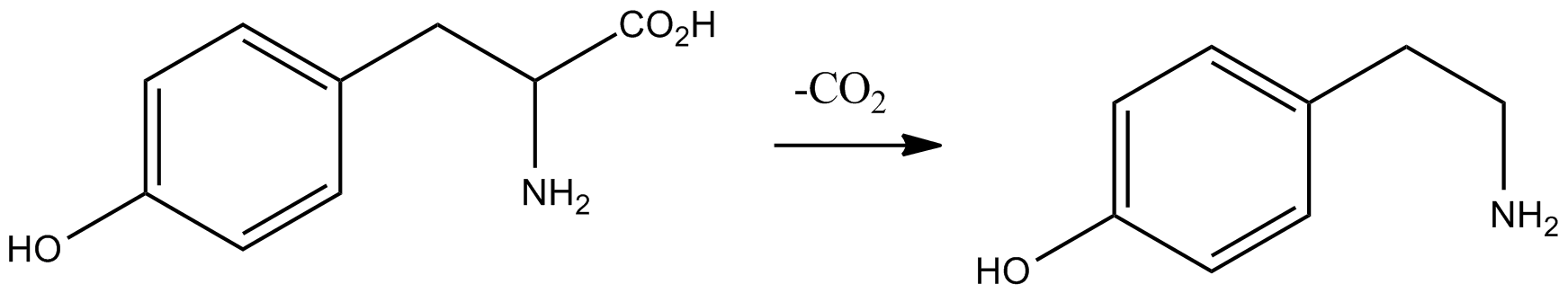
Meta-Tyramine
''meta''-Tyramine, also known as ''m''-tyramine and 3-tyramine, as well as 3-hydroxyphenethylamine, is an endogenous trace amine neuromodulator and a structural analog of phenethylamine. It is a positional isomer of ''para''-tyramine, and similarly to it, has effects on the adrenergic and dopaminergic systems. ''meta''-Tyramine is produced in humans via aromatic amino acid decarboxylase-mediated metabolism of ''meta''-tyrosine. meta-Tyramine can be metabolized into dopamine via peripheral or brain CYP2D6 enzyme An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...s in humans. See also * ''para''-Tyramine * 3-Methoxytyramine * 3-Hydroxy-''N'',''N''-dimethylphenethylamine (LSM-6) References Phenethylamines 3-Hydroxyphenyl compounds TAAR1 agonists Trace amines Nor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trace Amine
Trace amines are an endogenous group of trace amine-associated receptor 1 (TAAR1) agonists – and hence, monoaminergic neuromodulators – that are structurally and metabolically related to classical monoamine neurotransmitters. Compared to the classical monoamines, they are present in trace concentrations. They are distributed heterogeneously throughout the mammalian brain and peripheral nervous tissues and exhibit high rates of metabolism. Although they can be synthesized within parent monoamine neurotransmitter systems, there is evidence that suggests that some of them may comprise their own independent neurotransmitter systems. Trace amines play significant roles in regulating the quantity of monoamine neurotransmitters in the synaptic cleft of monoamine neurons with TAAR1. They have well-characterized presynaptic ''amphetamine-like'' effects on these monoamine neurons via TAAR1 activation; specifically, by activating TAAR1 in neurons they promote the release and preve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trace Amines
Trace amines are an endogenous group of TAAR1 agonist, trace amine-associated receptor 1 (TAAR1) agonists – and hence, monoaminergic neuromodulators – that are structurally and metabolically related to classical monoamine neurotransmitters. Compared to the classical monoamines, they are present in trace concentrations. They are distributed heterogeneously throughout the mammalian brain and peripheral nervous tissues and exhibit high rates of metabolism. Although they can be synthesized within parent monoamine neurotransmitter systems, there is evidence that suggests that some of them may comprise their own independent neurotransmitter systems. Trace amines play significant roles in regulating the quantity of monoamine neurotransmitters in the synaptic cleft of monoamine neurons with TAAR1. They have well-characterized presynaptic ''amphetamine-like'' effects on these monoamine neurons via TAAR1 activation; specifically, by activating TAAR1 in neurons they promote the releas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endogenous
Endogeny, in biology, refers to the property of originating or developing from within an organism, tissue, or cell. For example, ''endogenous substances'', and ''endogenous processes'' are those that originate within a living system (e.g. an organism or a cell). For instance, estradiol is an endogenous estrogen hormone A hormone (from the Ancient Greek, Greek participle , "setting in motion") is a class of cell signaling, signaling molecules in multicellular organisms that are sent to distant organs or tissues by complex biological processes to regulate physio ... produced within the body, whereas ethinylestradiol is an exogenous synthetic estrogen, commonly used in birth control pills. In contrast, '' exogenous substances'' and ''exogenous'' ''processes'' are those that originate from outside of an organism. References External links *{{Wiktionary-inline, endogeny Biology ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TAAR1 Agonists
Trace amine-associated receptor 1 (TAAR1) is a trace amine-associated receptor (TAAR) protein that in humans is encoded by the ''TAAR1'' gene. TAAR1 is a primarily intracellular amine-activated and G protein-coupled receptor (GPCR) that is primarily expressed in several peripheral organs and cells (e.g., the stomach, small intestine, duodenum, and white blood cells), astrocytes, and in the intracellular milieu within the presynaptic plasma membrane (i.e., axon terminal) of monoamine neurons in the central nervous system (CNS). TAAR1 is one of six functional human TAARs, which are so named for their ability to bind endogenous amines that occur in tissues at trace concentrations. TAAR1 plays a significant role in regulating neurotransmission in dopamine, norepinephrine, and serotonin neurons in the CNS; it also affects immune system and neuroimmune system function through different mechanisms. Endogenous ligands of the TAAR1 include trace amines, monoamine neurotransmitters, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenethylamines
Substituted phenethylamines (or simply phenethylamines) are a chemical class of organic compounds that are based upon the phenethylamine structure; the class is composed of all the derivative (chemistry), derivative compounds of phenethylamine which can be formed by replacing, or substitution reaction, substituting, one or more hydrogen atoms in the phenethylamine core structure with substituents. Phenylethylamines are also generally found to be central nervous system stimulants with many also being entactogens/empathogens, and hallucinogens. Structural classification The structural formula of any substituted phenethylamine contains a phenyl group, phenyl ring that is joined to an amino group, amino (NH) group via a two-carbon substituent, sidechain. Hence, any substituted phenethylamine can be classified according to the substitution of hydrogen atom, hydrogen (H) atoms on phenethylamine's phenyl ring, sidechain, or amino group with a moiety (chemistry), specific group of at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-Hydroxy-N,N-dimethylphenethylamine
3-Hydroxy-''N'',''N''-dimethylphenethylamine (developmental code name LSM-6) is a drug of the phenethylamine family which was under development for the treatment of mood disorders but was never marketed. It is the ''N'',''N''-dimethylated derivative of ''meta''-tyramine (3-hydroxyphenethylamine). The drug is a naturally occurring constituent of ''Limacia scanden Lour.'' and is described as an adrenergic and serotonergic agent. It may act as a monoamine releasing agent and/or reuptake inhibitor. LSM-6 has sympathomimetic effects. It was being developed in the 1990s in Malaysia. The drug reached the preclinical research stage of development prior to its discontinuation. See also * List of investigational antidepressants * Trichocereine (3,4,5-trimethoxy-''N'',''N''-dimethylphenethylamine) * Macromerine (β-hydroxy-3,4-dimethoxy-''N'',''N''-dimethylphenethylamine) * Dimethylamphetamine (''N'',''N''-dimethylamphetamine) * Gepefrine (3-hydroxyamphetamine) * Pholedrine Phole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-Methoxytyramine
3-Methoxytyramine (3-MT), also known as 3-methoxy-4-hydroxyphenethylamine, is a human trace amine and the major metabolite of the monoamine neurotransmitter dopamine. It is formed by the introduction of a methyl group to dopamine by the enzyme catechol-''O''-methyltransferase (COMT). 3-MT can be further metabolized by the enzyme monoamine oxidase (MAO) to form homovanillic acid (HVA), which is then typically excreted in the urine. Occurrence 3-Methoxytyramine occurs naturally in the prickly pear cactus (genus ''Opuntia''), and is in general widespread throughout the Cactaceae. It has also been found in crown gall tumors on ''Nicotiana'' sp. In humans, 3-methoxytyramine is a trace amine that occurs as a metabolite of dopamine. Biological activity Originally thought to be physiologically inactive, 3-MT was subsequently found to act as an agonist of the rodent and human TAAR1. 3-MT can induce weak hyperlocomotion in mice and this effect is partially attenuated in TAAR1 kno ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tyramine
Tyramine ( ) (also spelled tyramin), also known under several other names, is a naturally occurring trace amine derived from the amino acid tyrosine. Tyramine acts as a catecholamine releasing agent. Notably, it is unable to cross the blood-brain barrier, resulting in only non-psychoactive peripheral sympathomimetic effects following ingestion. A hypertensive crisis can result, however, from ingestion of tyramine-rich foods in conjunction with the use of monoamine oxidase inhibitors (MAOIs). Occurrence Tyramine occurs widely in plants and animals, and is metabolized by various enzymes, including monoamine oxidases. In foods, it often is produced by the decarboxylation of tyrosine during fermentation or decay. Foods that are fermented, cured, pickled, aged, or spoiled have high amounts of tyramine. Tyramine levels go up when foods are at room temperature or go past their freshness date. Specific foods containing considerable amounts of tyramine include: * Strong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts include Ribozyme, catalytic RNA molecules, also called ribozymes. They are sometimes descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP2D6
Cytochrome P450 2D6 (CYP2D6) is an enzyme that in humans is encoded by the ''CYP2D6'' gene. ''CYP2D6'' is primarily expressed in the liver. It is also highly expressed in areas of the central nervous system, including the substantia nigra. CYP2D6, a member of the cytochrome P450 mixed-function oxidase system, is one of the most important enzymes involved in the metabolism of xenobiotics in the body. In particular, CYP2D6 is responsible for the metabolism and elimination of approximately 25% of clinically used drugs, via the addition or removal of certain functional groups – specifically, hydroxylation, demethylation, and dealkylation. CYP2D6 also activates some prodrugs. This enzyme also metabolizes several endogenous substances, such as N,N-Dimethyltryptamine, hydroxytryptamines, neurosteroids, and both ''m''-tyramine and ''p''-tyramine which CYP2D6 metabolizes into dopamine in the brain and liver. Considerable variation exists in the efficiency and amount of C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dopamine
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor chemical, L-DOPA, which is synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons (nerve cells) to send signals to other nerve cells. The brain includes several distinct dopamine pathways, one of which plays a major role in the motivational component of reward-motivated behavior. The anticipation of most types of rewards increases the level of dopamine in the brain, and many addictive drugs increase dopamine release or block its reuptake into neurons following release. Other brain dopamine pathways are involved in motor control and in controllin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |